Lecture 15
Nuclear Chemistry
Shaun Williams, PhD
Radioactivity
- Radiation
- Energy that comes from a source and travels through matter or space
- Two types of radiation:
- Electromagnetic - Includes light, gamma rays, and X-rays
- Particulate - Mass given off from unstable atoms with the energy of motion
- Ionizing radiation
- Radiation of either type that can produce charged particles in matter
Radioactivity
- Radioactive decay
- The spontaneous emission of electromagnetic or other types of radiation
- Radioactive atoms
- Unstable atoms that give off excess matter, energy, or both as ionizing radiation
Nucleons
- General term used to describe nuclear particles, protons, and neutrons
- Remember:
- ZZ signifies the atomic number, the number of protons in the nucleus of an atom
- NN signifies the neutron number, the number of neutrons in the nucleus of an atom
- Sum of NN and ZZ is AA (N+Z=AN+Z=A), the mass number
Nuclides
- Remember, isotopes are:
- Atoms with the same atomic number ZZ, but different neutron numbers NN and mass numbers AA
- Nuclides
- Isotopes that exist for a measurable length of time and have a defined energy state
- An atom of a particular atomic number, mass number and neutron number
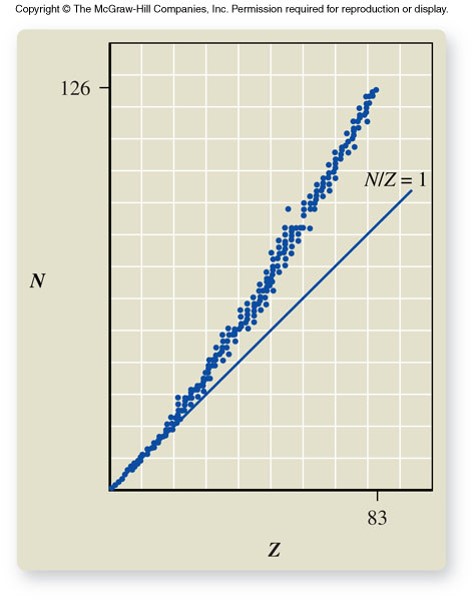
Band of Stability
- Of the more than 3,000 nuclides known, about 250 are stable
- The rest decompose over a period of time, emitting radiation in the process of creating new nuclides
- The stable nuclides have approximately equal numbers of protons and neutrons (N/ZN/Z ratio = 1) in the lighter elements (ZZ = 1 to 20) and more neutrons than protons in the heavier elements (N/ZN/Z ratio > 1).
Radiation
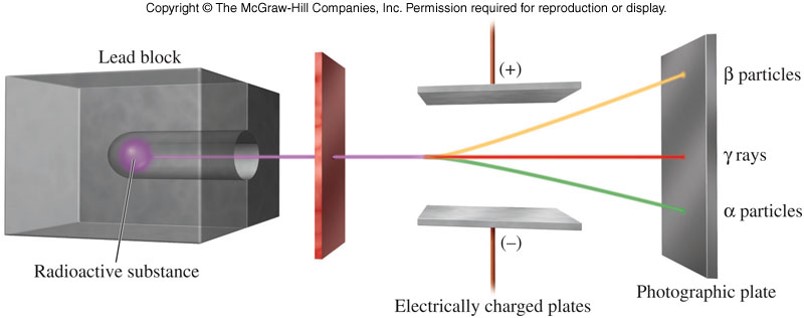
- In a nuclear reaction, an emission of radiation usually accompanies changes in the composition of the nucleus.
- Natural radiation associated with radioactive decay can be placed into three classes:
- Alpha particles
- Beta particles
- Gamma rays
Properties of Types of Radiation
| Type | Notation | Mass | Charge | Penetration into Al |
|---|---|---|---|---|
| Alpha | 42α,42He2+42α,42He2+ | 4 | 2+ | 0.01 mm |
| Beta (electron) | 0−1β−0−1β− | ~0 | 1- | 0.5-1.0 mm |
| Beta (positron) | 01β+01β+ | ~0 | 1+ | (Reacts with electrons) |
| Gamma | γγ | 0 | 0 | 50-110 mm |
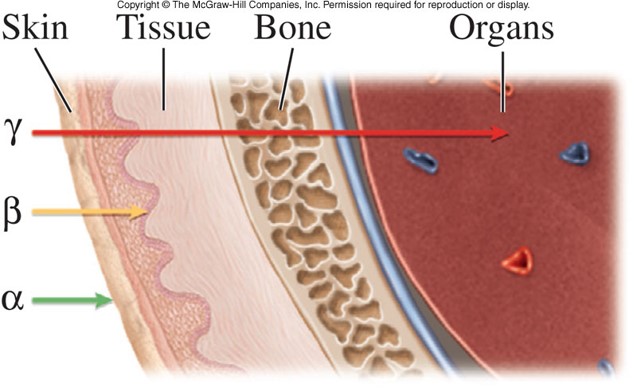
Types of Radiation
- Alpha particles
- Nuclei of helium-4 atoms
- Contain 2 protons and 2 neutrons
- Least harmful to animal and human tissue
- Gamma rays
- High energy electromagnetic radiation: energy without charge or mass
- Highest energy and most penetrating type of radiation
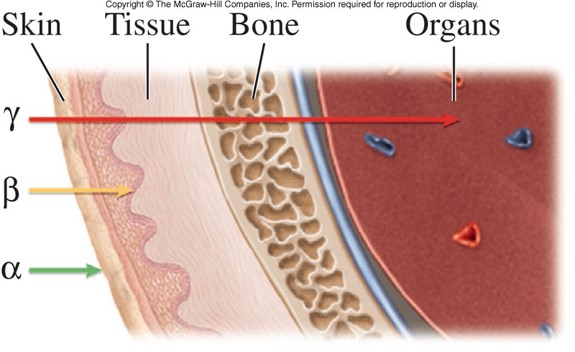
Types of Radiation (cont.)
- Beta particles
- Small, charged particle that can be emitted from unstable atoms at speeds approaching the speed of light
- Penetrate through skin into tissue
- 2 types of beta particles:
- Positron - Same mass as an electron with an opposite charge
- Electron
Nuclear Reactions
- Two conditions must be met to balance a nuclear equation:
- Conservation of mass number
- Conservation of nuclear charge (atomic number)
- Examples: 23290Th→22888Ra+42α23290Th→22888Ra+42α 23190Th→23191Pa+0−1β−23190Th→23191Pa+0−1β− 23892U+42α→23994Pu+310n23892U+42α→23994Pu+310n
Alpha Particle Emission
- When a nucleus emits an alpha particle, it loses 2 protons and 2 neutrons, so its atomic number decreases by 2 and its mass number decreases by 4. 23290Th→22888Ra+42α23290Th→22888Ra+42α
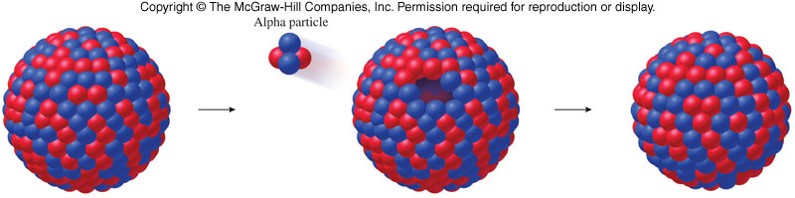
Beta Particle (Electron) Emission
- When a nucleus emits a beta particle (electron), its atomic number increases by 1 and its mass number remains unchanged. 23190Th→23191Pa+0−1β−23190Th→23191Pa+0−1β−
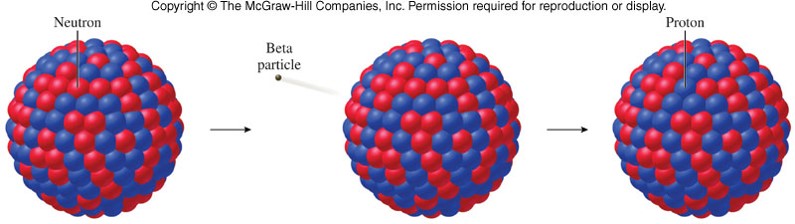
Beta Particle (Positron) Emission
- When a nucleus emits a beta particle (positron), its atomic number decreases by 1 and its mass number remains unchanged. 2312Mg→2311Na+01β+2312Mg→2311Na+01β+
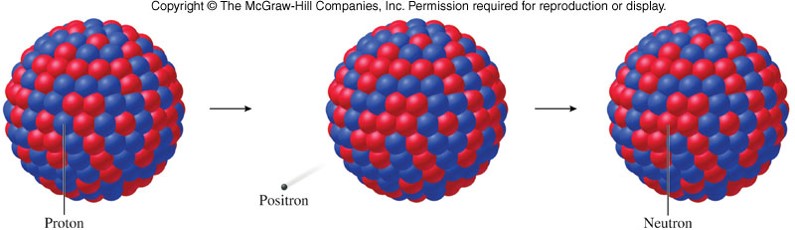
Electron Capture
- A proton and an electron combine to form a neutron. The mass number stays the same, but the atomic number decreases by 1.
- Very few nuclides undergo this transformation. 74Be+0−1e−→73Li74Be+0−1e−→73Li
Gamma Ray Emission
- In all nuclear reactions, the nucleus changes from a state of higher energy to a state of lower energy.
- Gamma rays are pure electromagnetic energy.
- Results in no change in mass or atomic number. 99mTc→99Tc+γ99mTc→99Tc+γ
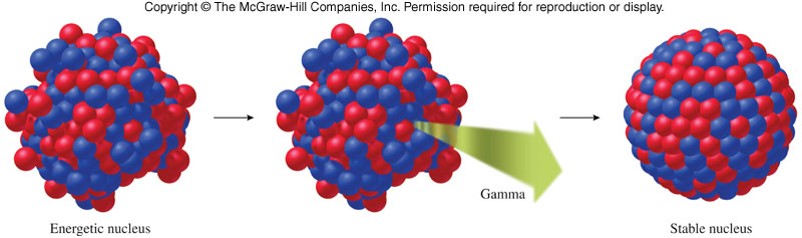
Nuclear Bombardment Reactions
- Nuclei are hit with a beam of nuclei or nuclear particles to trigger a nuclear reaction
- Occurs when a nuclear reaction is not spontaneous and is produced intentionally by artificial means
- Used to synthesize transuranium elements, those following uranium on the periodic table
- Some examples: 23892U+10n→23992U→23993Np+0−1β−23892U+10n→23992U→23993Np+0−1β− 9742Mo+21H→9743Tc+210n9742Mo+21H→9743Tc+210n 20983Bi+42α→21185At+210n20983Bi+42α→21185At+210n
Particle Accelerators
- Particle accelerators are used for nuclear bombardment reactions.
- The synchroton, perhaps the most successful accelerator, uses a circular path for the accelerating particles.

Sponeaneous Nuclear Decay Reactions
- The tendency for the neutron/proton (N/ZN/Z) ratio to move toward the band of stability, explains the nuclear reactions of naturally radioactive nuclides.
- For every process except γγ emission, the change that occurs for an unstable nuclide takes it closer to the observed band of stability.
- Radioactive nuclides convert spontaneously over time to form stable nuclides.
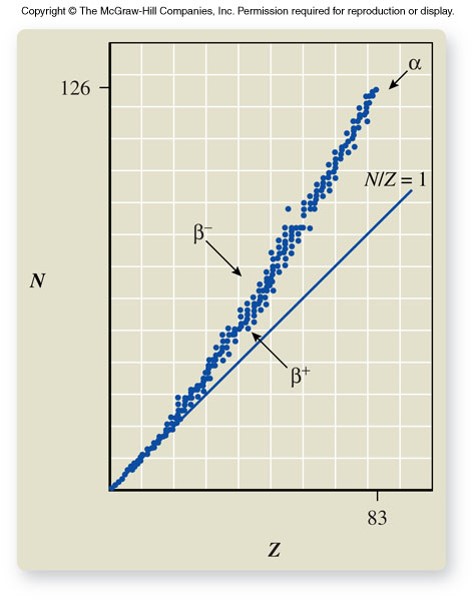
Nuclear Instability
| Reason for Nuclear Instability | Radioactive Process | Emitted Radiation | Change in N/ZN/Z Ratio |
|---|---|---|---|
| Excess Mass | Alpha decay | 42α42α | Slight increase |
| N/ZN/Z too high | Beta decay | 0−1β−0−1β− | Decrease |
| N/ZN/Z too low | Positron emission | 01β+01β+ | Increase |
| N/ZN/Z too low | Electron capture | - | Increase |
| Energetically excited | Gamma emission | γγ ray | None |
Radioactive Decay Series
- In heavier elements, often the product of radioactive decay is itself radioactive.
- In such cases, a series of alpha and beta decay steps ultimately leads to a stable nuclide.
- Accounts for most of the radioactive decay among elements 83 through 92.
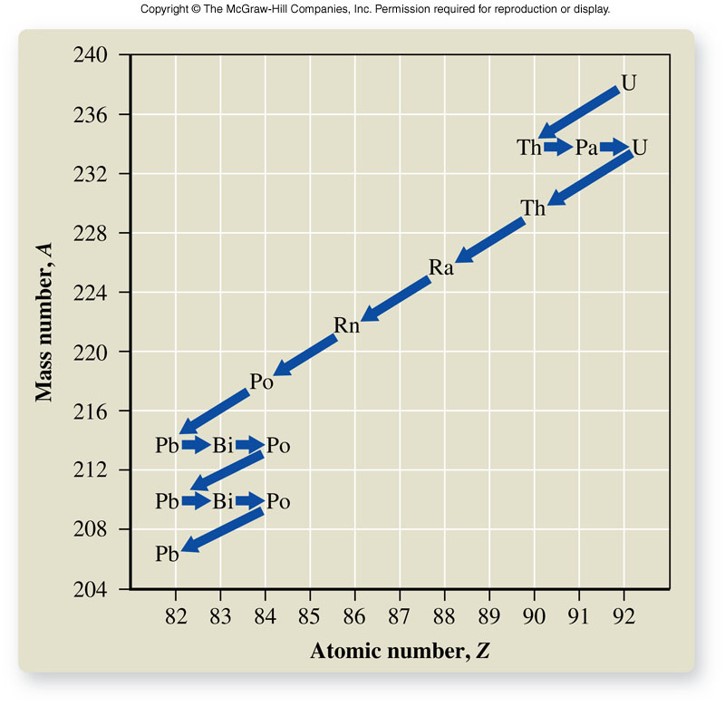
Rates of Radioactive Decay
Detecting Radiation
- Various instruments have been developed to give speedier and more accurate measures of radiation intensity:
- Geiger-Muller counter
- Scintillation counter
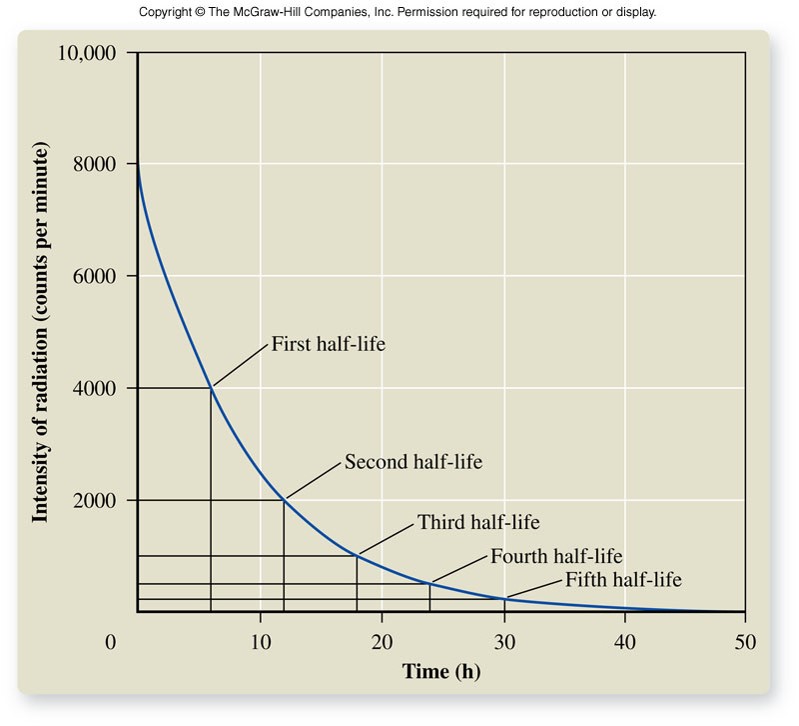
Half-Life
- The time required for half of a sample of a nuclide to decay to a different nuclide
- It takes the same time for a fresh sample to decay to one-half the original number of atoms of that nuclide as it does one-half to decay to one-fourth and so on.
- The shorter the half-life of a nuclide, the more intense the radiation that it emits.
Archeological Dating
- Radio-carbon dating
- Using carbon-14 to measure time on an archeological scale
- As long as a plant or animal is alive, its carbon-14 content should match that in the atmosphere
- After it dies, its carbon-14 content decreases through beta decay: 146C→147N+0−1β−146C→147N+0−1β−
- The half-life of the process is 5730 years.
Medical Applications of Isotopes
- Many medical applications exists that use radioactivity:
- Power generators
- Example: 238Pu238Pu is used to power pacemakers
- Medical diagnoses
- Radioactive nuclides are used as tracers to track movements of substances in chemical or biological systems
- Example: 99mTc99mTc is used to help doctors locate tumors
- Power generators
Medical Applications
- Positron Emission Topography
- A PET scan detects abnormalities in living tissues without disrupting the tissue.
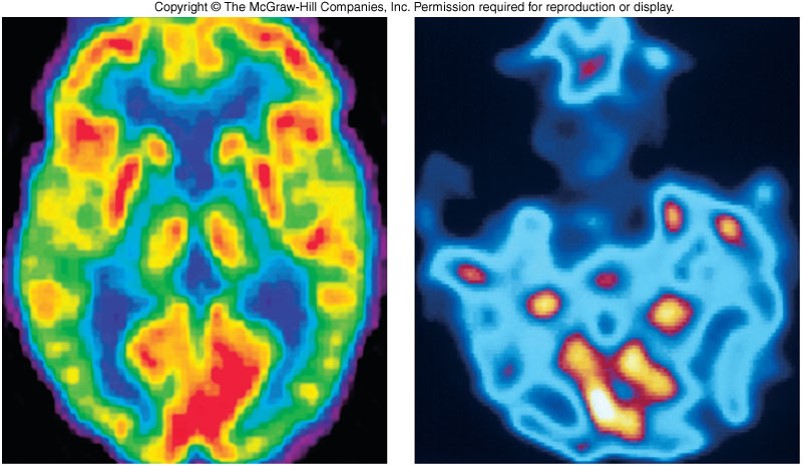
Medical Applications (cont.)
- Cancer therapy
- Radioactive nuclides, in much higher doses than those used for imaging, are used to treat cancerous tumors
- Cancer cells absorb nutrients containing gamma-emitting components, the gamma radiation becomes concentrated in the cancerous cells, destroying them in greater numbers than normal cells.
- Examples: 131I131I destroys thyroid tumors, 198Au198Au used to treat lung cancer, 32P32P used for eye tumors
Biological Effects of Radiation
- Radiation can have one of four effects on the functioning of a cell:
- The radiation can pass through the cell with no damage.
- The cell can absorb the radiation and be damaged, but it can subsequently repair the damage and resume normal functioning.
- The cell can be damaged so severely that it cannot repair itself. New cells formed from this cell will be abnormal. This mutant cell can ultimately cause cancer if it continues to proliferate.
- The cell can be so severely damaged that it dies.
Biological Effects of Radiation - Radon
- A rare noble gas which has also been implicated as a possible cause of lung cancer
- Accumulates in houses from particular kinds of soils or rock strata
The Fear of Nuclear Energy...
Before and After...
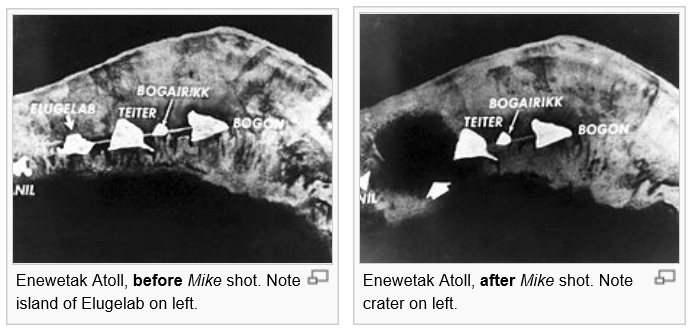
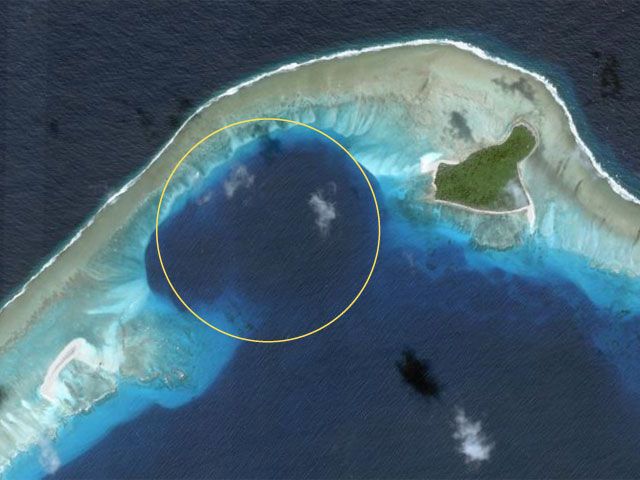
Comparison
Nuclear Energy
Fission
- Splitting of a heavy nucleus into two or more lighter nuclei and some number of neutrons
- Example: 23592U+10n→9236Kr+14156Ba+310n23592U+10n→9236Kr+14156Ba+310n 23592U+10n→9038Sr+14354Xe+310n23592U+10n→9038Sr+14354Xe+310n 23592U+10n→9440Zr+14058Ce+210n+60−1β−23592U+10n→9440Zr+14058Ce+210n+60−1β−
Fission of Uranium-235
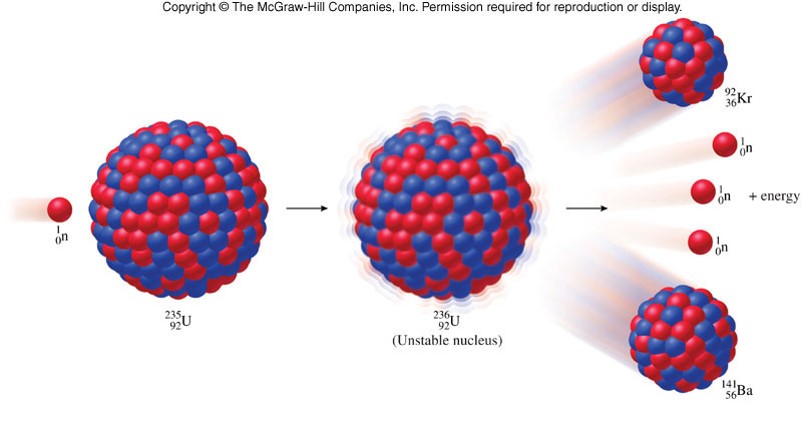
Chain Reactions
- A reaction in which the product of one step is the reactant in another step
- In order for a chain reaction to sustain itself, the amount and shape of the sample of fissionable material must be such that the neutrons will not escape due to energy that is higher than optimum for inducing further fission
- A chain reaction should maintain a constant rate
- Critical mass - The smallest amount of fissionable material necessary to support a continuing chain reaction
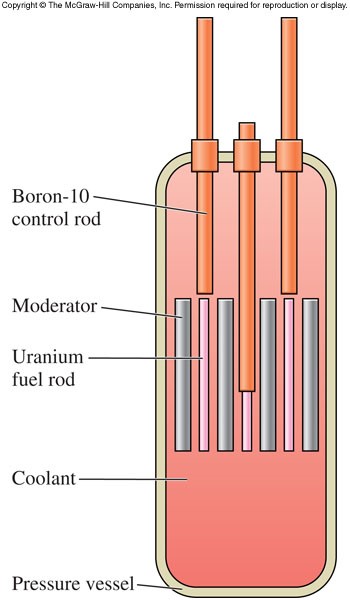
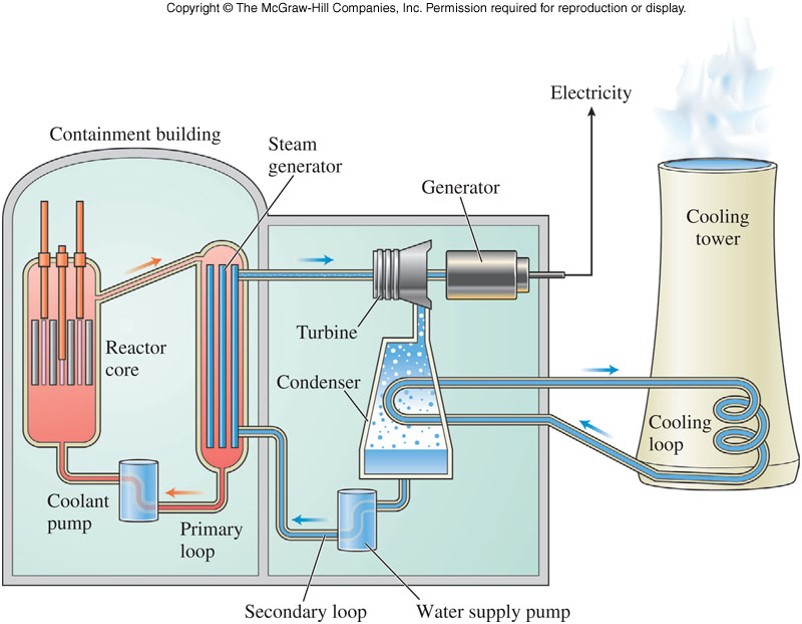
Fission Reactors
- Nuclear power plants use fission to produce electric energy
- If the chain reaction is going too quickly, movable control rods made of these elements are inserted into a core of uranium fuel in fission reactors
Fusion Reactions
- Combination of light nuclei to form heavier nuclei
- A major fusion reaction occurs continuously in the Sun and other stars: 411H→42He+201β+ This process occurs in several steps: 11H+11H→21H+01β+ 11H+21H→32He 11H+32He→42He+01β+
Fusion Reaction Terms
- Ignition temperature - Temperature required to initiate a fusion reaction
- Breeder reactors - A reactor that produces fuel that can be used in other reactors
- Plasma
- An ionized gas that must be created and controlled at temperatures of about 108K
- Melts most container material
- Until recently, fusion in reactors required more energy than was given off
- In order to achieve fusion, the gaseous reactants must be condensed to a small volume at high temperatures.
1 / 38