Chapter 11
Solutions
Shaun Williams, PhD
The Composition of Solutions
- A solution is:
- A homogeneous mixture with uniform composition throughout
- Composed of:
- Solute
- Substance being dissolved
- Usually present in the lesser amount
- Solvent
- Substance doing the dissolving
- Usually present in the greater amount
- Solute
Making a Solution

- What is the solute?
- What is the solvent?
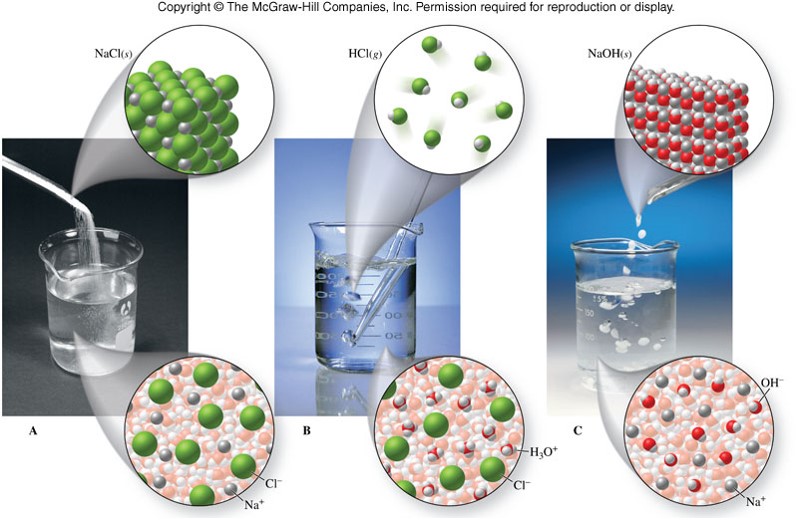
Various Solutions
Solubility
- How readily or completely a solute dissolves in a solvent
- A soluble ionic compound dissociates into ions when dissolved in water.
- Insoluble compounds remain mostly undissociated in their ionic, crystalline structure.
- The solubilities for ionic compounds do not follow a predictable pattern
- Must be recalled from experimental data
- Solubility rules are listed on the next slide
Solubility Rules
| # | Ions | Rules |
|---|---|---|
| 1 | Na+,K+,NH+4 | Most salts of sodium, potassium, and ammonium ions are soluble. |
| 2 | NO−3 | All nitrates are soluble. |
| 3 | SO2−4 | Most sulfates are soluble. Exceptions: BaSO4, SrSO4, PbSO4, CaSO4, Hg2SO4, & Ag2SO4 |
| 4 | Cl−,Br−,I− | Most chlorides, bromides, and iodides are soluble. Exceptions: AgX, Hg2X, PbX2, & HgI2 (X=Cl,Br,orI) |
| 5 | Ag+ | Silver salts are insoluble. |
| 6 | O2−,OH− | Oxides and hydroxides are insoluble. Exceptions: Ba(OH)2 & Ca(OH)2 (somewhat soluble) |
| 7 | S2− | Sulfides are insoluble. Exceptions: Salts of alkaline metals and alkaline earth metal ions |
| 8 | CrO2−4 | Most chromates are insoluble. Exceptions: Salts of Mg2+, Ca2+, Al3+, and Ni2+ |
| 9 | CO2−3,PO2−4,SO2−3,SiO2−3 | Most carbonates, phosphates, sulfites, and silicates are insoluble. |
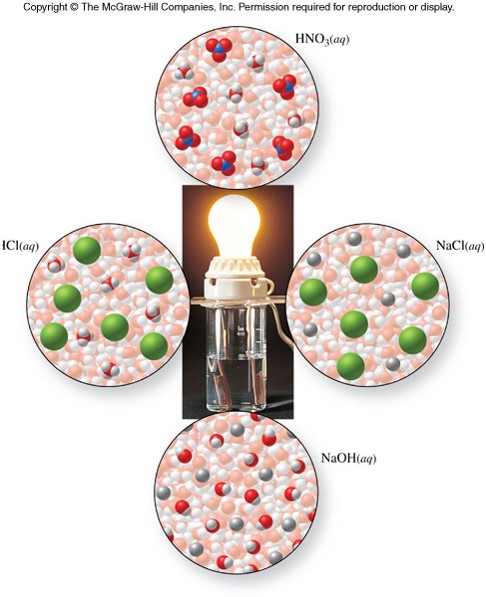
Electrolytes
- A solution that conducts electricity
- Two kinds of electrolytes:
- Strong electrolyte
- A solute that dissociates completely into ions in aqueous solution
- Weak electrolyte
- A solute that dissociates partially into ions in aqueous solution
- Nonelectrolyte
- A solute that does not dissociate into ions in aqueous solution
- Strong electrolyte
Examples of Electrolytes
Like Dissolves Like
- When the bonding in a solvent is similar to the bonding in a solute, then the solute will dissolve in the solvent.
- Polar covalent solvents dissolve polar covalent solutes.
- Nonpolar covalent solvents dissolve nonpolar covalent solutes.
- See the table below
| Solute | Solvent | |
|---|---|---|
| Polar | Nonpolar | |
| Ionic | Soluble | Insoluble |
| Polar | Soluble | Insoluble |
| Nonpolar | Insoluble | Soluble |
The Solution Process
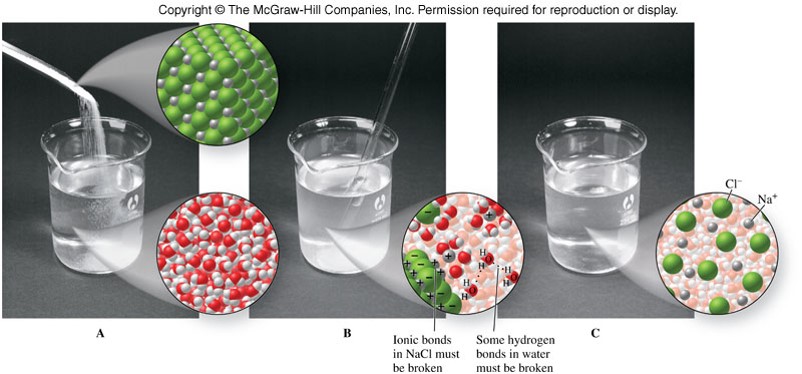
Solution Process
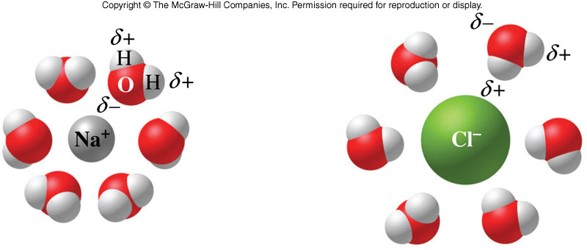
- Ion-dipole force
- An attraction between an ion and a polar molecule
- When an ionic compound dissolves in water:
- Ionic bonds in the solute break.
- Hydrogen bonds between water molecules break.
- Ion-dipole forces form between ions and water molecules.
The Energetics of the Solution Process
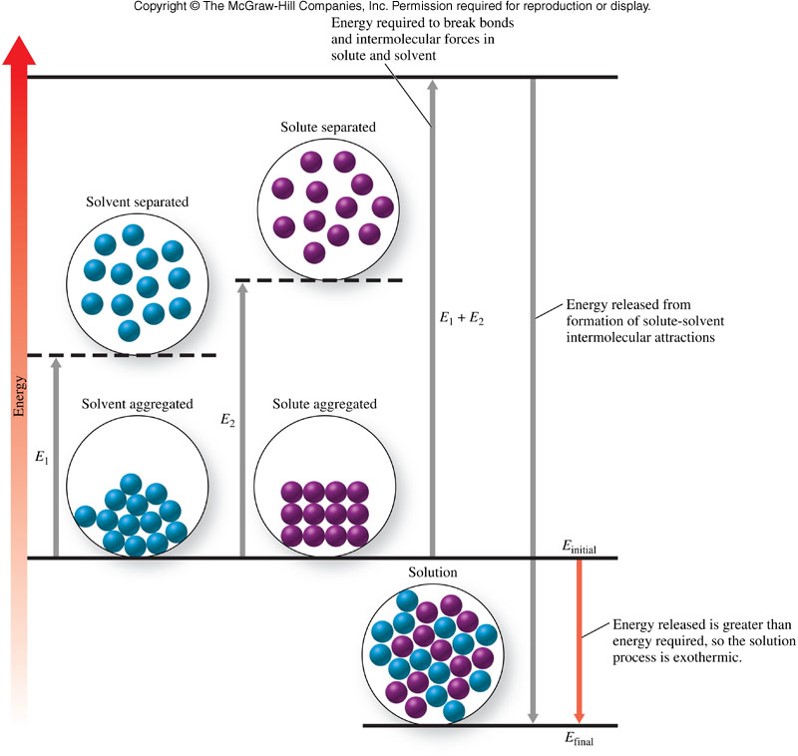
Heat of Solution
- The difference between the energy required for separation of solute and solvent and the energy released upon formation of ion-dipole interactions
- When the solvation process provides more energy than is needed to separate the pure solvent and solvent particles, the heat of solution is negative, and the overall dissolving process is exothermic.
Endothermic Solution Process
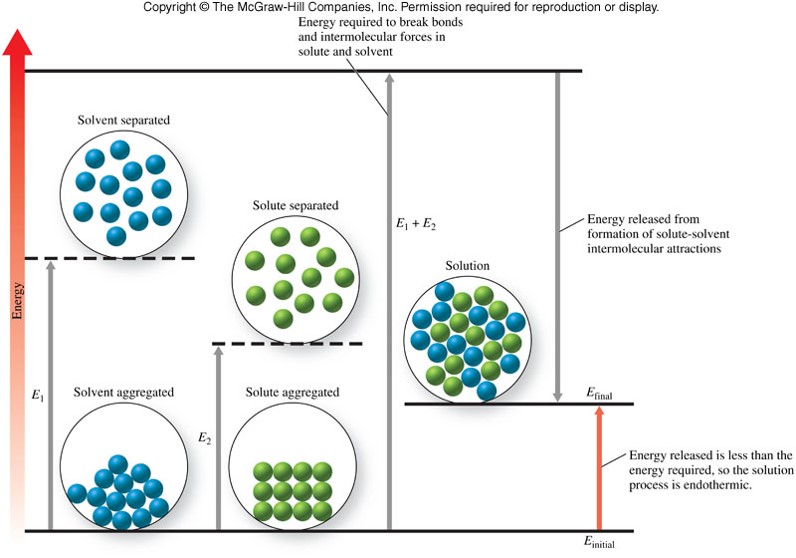
Solution Process for Nonpolar-Nonpolar Interactions
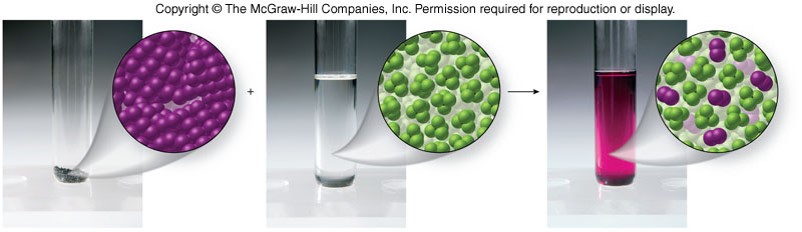
Entropy
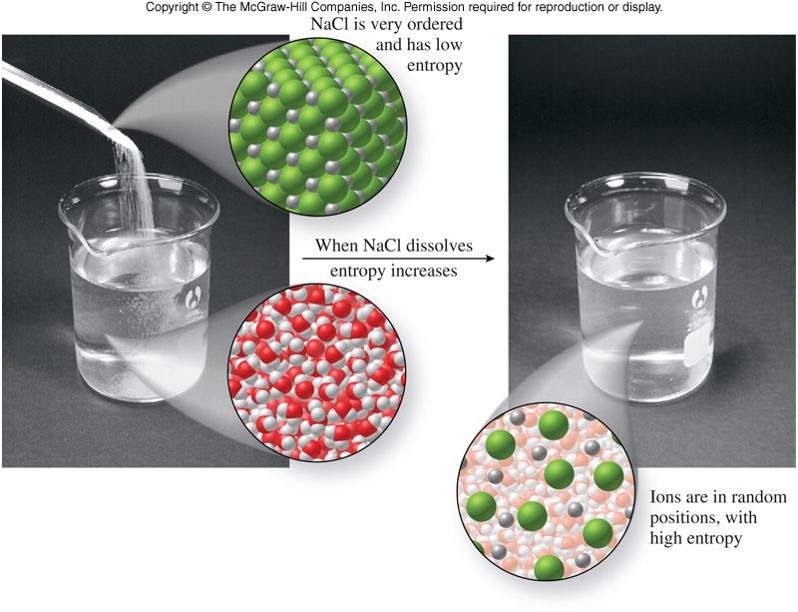
- A measure of the tendency for matter to become disordered or random in its distribution
- Matter spontaneously changes from a state of order to a state of randomness, unless energy changes oppose it.
- Solutions form because the makeup of the solution naturally tends toward disorganization.
Entropy in Dissolving
Factors that Affect Solubility
- Three factors affect solubility:
- Structure
- Temperature
- Pressure
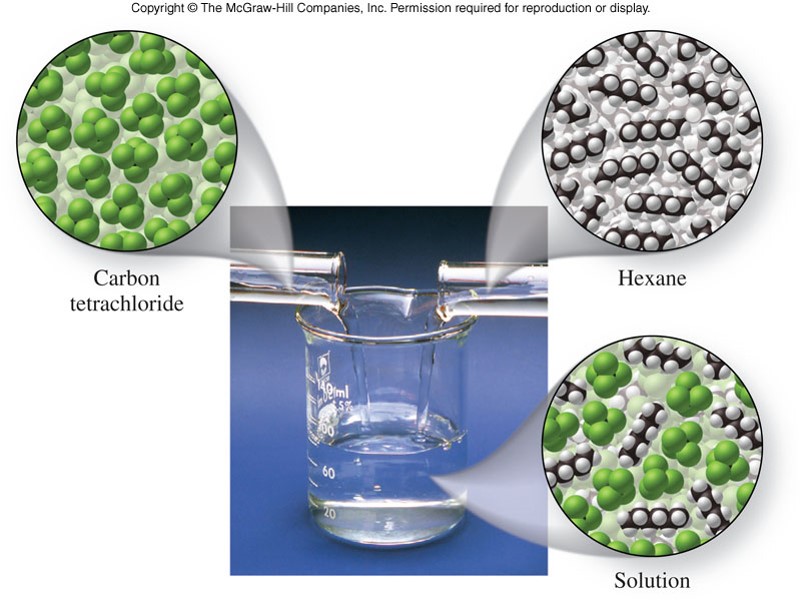
Structure
- For a solid to be soluble in a given solvent, the energy released by the solvation process must compensate for all of the energy required to break up the forces at work both within the solute and the solvent.
- Entropy is also a factor
- The solubility (or miscibility) of liquids in other liquids depends largely on the polarity of the solute and solvent molecules.
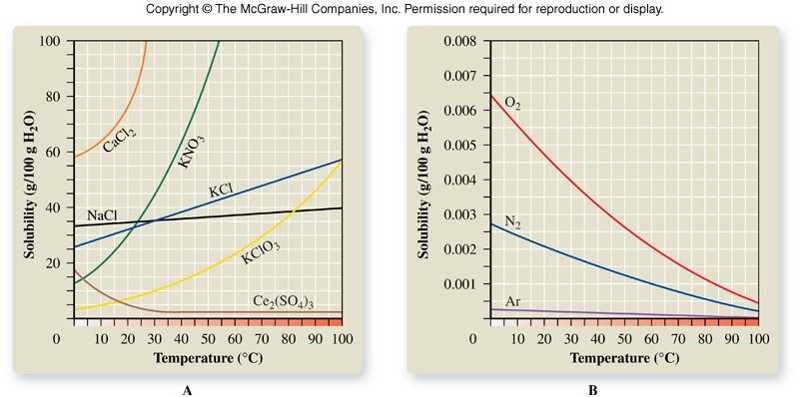
Temperature
- Affects the solubility of most substances in water
- Most ionic solids are more soluble in water at higher temperatures
- Gases are less soluble as temperature increases
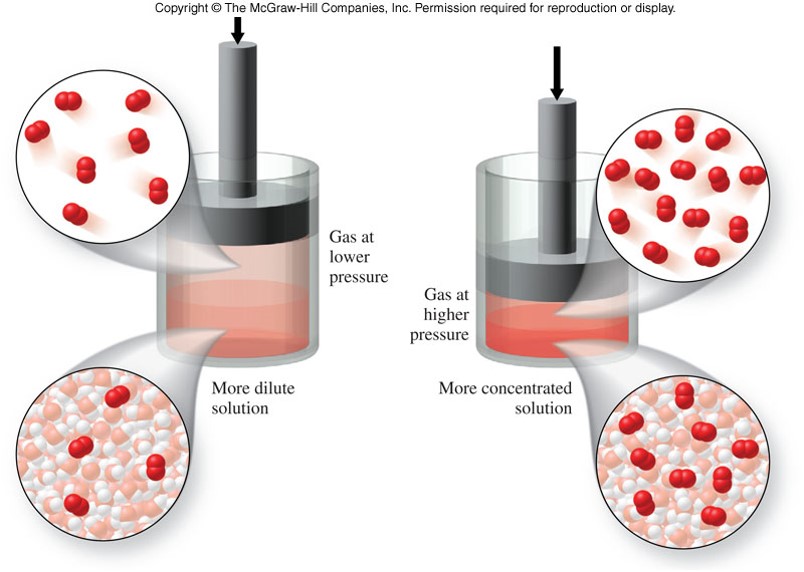
Pressure
- Affects gases but not liquids or solids
- The solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid
- An increase in pressure results in an increase in solubility of a gas
Pressure Effect in Action
Measuring Concentrations of Solutions
- Concentration
- The relative amounts of the solutes and solvent that make up the solution Concentration=amount of soluteamount of solution (or solvent)
- Solubility
- The ratio that identifies the maximum amount of a solute that will dissolve in a particular solvent to form a stable solution under specified conditions
- A measure of maximum concentration
- Three types of concentrated solution:
- Saturated solution
- Unsaturated solution
- Supersaturated solution
Saturated Solution
- A solution that contains the maximum possible amount of solute
- When this type of solution forms, a dynamic equilibrium is established.
- Undissolved solute continues to dissolve, but at the same time, previously dissolved solute is deposited from solution.
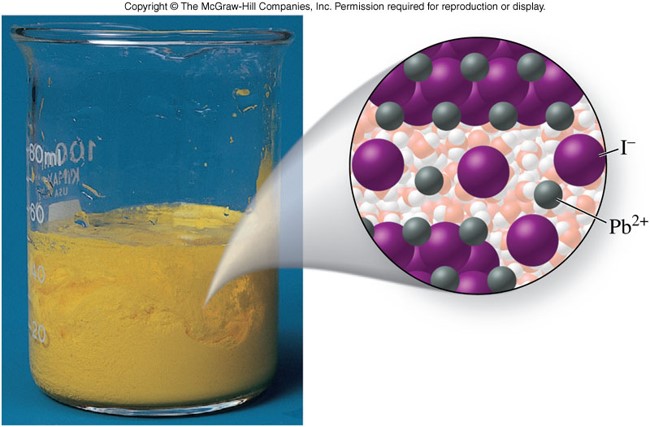
Supersaturated Solution
- A solution that contains more than the maximum possible amount of solute
- Unsaturated solution
- A solution that contains less than the maximum possible amount of solute

Concentration Units
| Unit | Definition |
|---|---|
| Percent by mass | grams of solutegrams of solution×100% |
| Percent by volume | volume of solutevolume of solution×100% |
| Mass/volume percent | grams of solutevolume of solution×100% |
| Parts per million | grams of solutegrams of solution×106 |
| Parts per billion | grams of solutegrams of solution×109 |
| Molarity (M) | moles of soluteliters of solution |
| Molality (m) | moles of solutekilograms of solvent |
Percent by Mass
- Is calculated by dividing the solute mass by the mass of the solution and multiplying it by 100% Percent by mass=mass (solute)mass (solution)×100%
- To find the mass of the solution, we can add the mass of the solute to the mass of the solvent.
Percent by Volume
- Is calculated by dividing the solute volume by the solution volume and multiplying by 100% Percent by volume=volume (solute)volume (solution)×100%
- The volume of the solution must be measured. Volumes are not additive like masses.
Mass/Volume Percent
- Is calculated by dividing the solute mass by the volume of solution and multiplying by 100% Mass/Volume Percent=grams (solute)volume (solution)×100%
- Example:
- The concentration of NaCl in a saline bag is 0.9%. Therefore, for every 100 mL of solution, the mass of NaCl present is 0.9 g.
Parts per Million and Billion
- Parts per million
- Is calculated by dividing the mass of solute by the mass of solution and multiplying by 1 million (106) Parts per million=mass (solute)mass (solution)×106
- Parts per billion
- Is calculated by dividing the mass of solute by the mass of solution and multiplying by 1 billion (109) Parts per billion=mass (solute)mass (solution)×109
Molarity
- The ratio of moles of solute to the volume of solution in liters Molarity(M)=moles (solute)liters (solution)
- Example:
- A solution contains 117 g of potassium hydroxide (KOH) dissolved in sufficient water to give a total volume of 2.00 L. The molar mass of KOH is 56.11 g/mol. What is the molarity of the aqueous potassium hydroxide solution?
Molality
- If the temperature of a solution increases, the molarity of the solution changes because the volume changes.
- Molality does not change with temperature because it divides moles of solute with the mass of solution. Molality(m)=moles (solute)kg (solvent)
Quantities for Reactions That Occur in Aqueous Solution
Precipitation Reactions
- The cation from one reactant combines with the anion from another reactant to form an insoluble compound, called a precipitate.
- Example
- When a solution of lead(II) nitrate is mixed with a solution of potassium chromate, a yellow precipitate forms according to the equation: Pb(NO3)2(aq)+K2CrO4(aq)→2KNO3(aq)+PbCrO4(s) What volume of 0.105 M lead(II) nitrate is required to react with 100.0 mL of 0.120 M potassium chromate? What mass of PbCrO4 solid forms?
Acid-Base Titrations
- Titration
- The process of determining the concentration of one substance in solution by reacting it with a solution of another substance that has a known concentration
- Equivalence point
- The point in a titration at which the moles of the acid equal the moles of the base
- End point
- The point in a titration where the indicator changes color
- Slightly different than the equivalence point, but a good estimation
Acid-Base Titration Observation

Colligative Properties
- A property that does not depend on the identity of a solute in solution
- Vary only with the number of solute particles present in a specific quantity of solvent
- 4 colligative properties:
- Osmotic pressure
- Vapor pressure lowering
- Boiling point elevation
- Freezing point depression
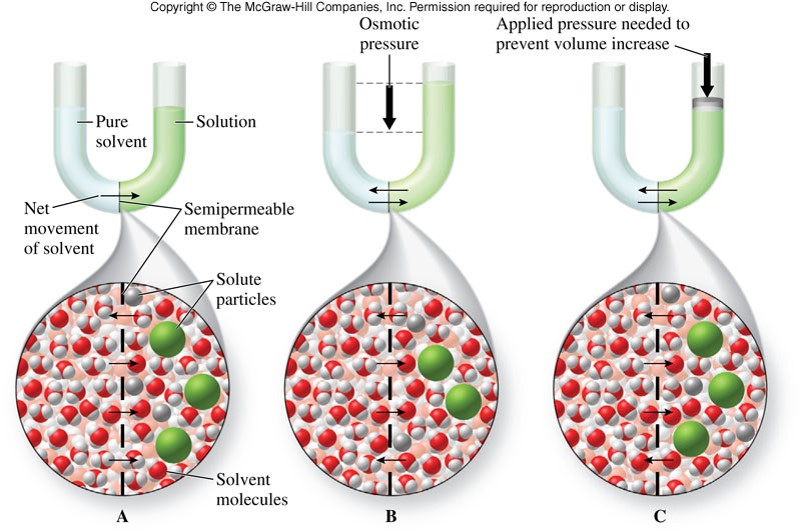
Osmotic Pressure
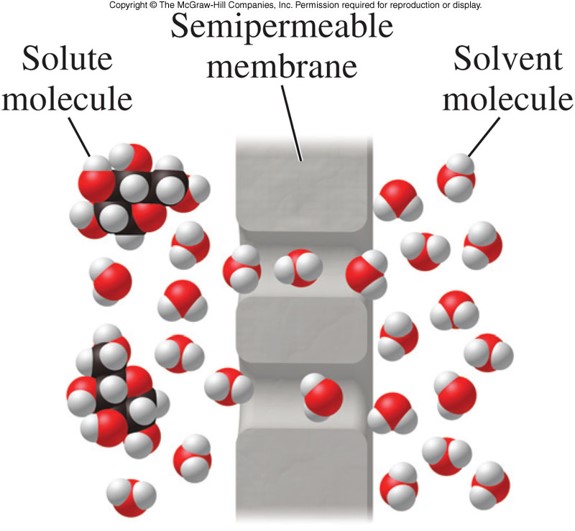
- Osmosis
- A process in which solvent molecules diffuse through a barrier that does not allow the passage of solute particles
- The barrier is called a semipermeable membrane.
- A membrane that allows the passage of some substances but not others
- Osmotic Pressure
- Pressure that can be exerted on the solution to prevent osmosis
Measuring Osmotic Pressure
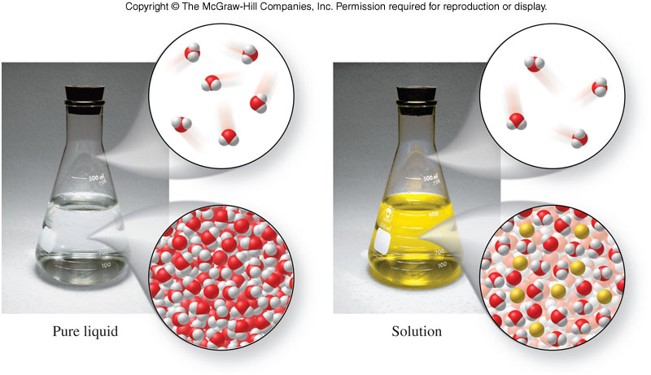
Vapor Pressure Lowering
- Solutes come in 2 forms:
- Volatile - Solutes that readily form a gas
- Nonvolatile - Solutes that DO NOT readily form a gas
- Generally, the addition of a solute lowers the vapor pressure of a solution when compared to the pure solvent.
Effect of Vapor Pressure Lowering

Phase Diagram
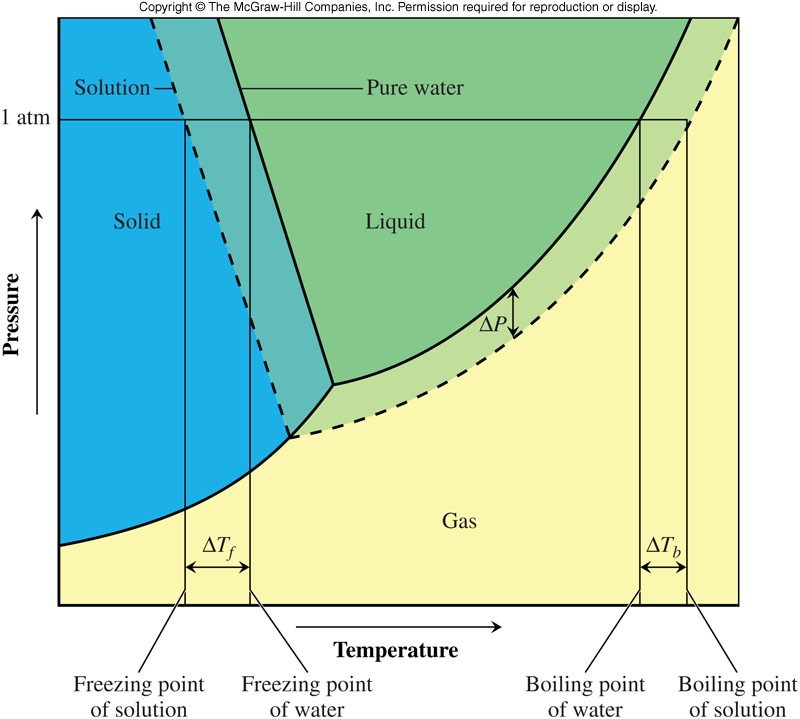
Boiling Point Elevation
- The addition of solute affects the boiling point because it affects the vapor pressure.
- The boiling point is raised with the addition of solute in comparison to the pure solvent.
- An equation that gives the increase in boiling point: ΔTb=Kbm where ΔTb is the increase in temperature from the pure solvent's boiling point, Kb is the boiling point constant, which is characteristic of a particular solvent, and m is the molality (moles of solute per kg of solution)
Freezing Point Depression
- The freezing point is lowered with the addition of solute in comparison to the pure solvent.
- An equation that gives the decrease in freezing point: ΔTf=Kfm where ΔTf is the decrease in temperature from the pure solvent's freezing point, Kf is the freezing point constant, which is characteristic of a particular solvent, and m is the molality (moles of solute per kg of solution)
1 / 43