Acid-Base Chemistry
Shaun Williams, PhD
Brønsted and Lewis Acids and Bases
- The are three major classifications of substance knows as acids and bases
- The Arrhenius definition states that an acid produces H+ in solution and a base produced OH−.
- This was proposed by Svante Arrhenius in 1883
- In acid-base chemistry, the hydrogen ion is often written as proton,H+ but it usually exists in a hydrated form such as H3O+, H5O+2, H7O+3 (these are collectively called the hydronium ion) H++3H2O→H3O++2H2O⇌H5O+2+H2O⇌H7O+3
The Arrhenius Theory of Acids and Bases
- Arrhenius acids are substances which produce hydrogen ions in solution.
- Arrhenius bases are substances which produce hydroxide ions in solution.
- This is the simplest acid-base theory
- This theory successfully describes how acids and bases neutralize each other
- There are issues with why certain acids and bases aren't predicted to be acids and bases according to this theory
The Brønsted-Lowry Theory of Acids and Bases
- A Brønsted-Lowry acid is a proton (hydrogen ion) donor.
- A Brønsted-Lowry base is a proton (hydrogen ion) acceptor.
- In this theory, acids release protons while bases accept those protons F−(aq)+H2O(l)⇌HF(aq)+OH−(aq)
Dissociation Reactions
- Acid dissociation: HA(aq)⇌A−(aq)+H+(aq) Ka=[A−][H+][HA]
- Base dissociation: B(aq)+H2O(l)⇌HB+(aq)+OH−(aq) Kb=[HB+][OH−][B]
Conjugate Acids and Bases
- One consequence of these equilibria is that every acid (HA) has a conjugate base (A−) and vice-versa
- For a given acid or base, these equilibria are linked by the water dissociation equilibrium H2O(l)⇌H+(aq)+OH−(aq) Kw=[H+][OH−]=1.00×10−14at25∘C
Strong and Weak Acids and Bases
- Acids and bases that completely dissociate are strong HClO4(aq)→H+(aq)+ClO−4(aq)
- Note that the arrow implies that the reaction runs to completion
- Weak acids and bases dissociate only slightly - they exist mostly undissociated HC2H3O2(aq)+H2O(l)⇌C2H3O−2(aq)+H3O+(aq)
- The strength of a conjugate acid/base varies inversely with the strength of its parent acid or base
A Common Misconception
- It is a common misconception that strong acids and bases have weak conjugates.
- For a strong acid or base, K≫1
- Since KaKb=Kw, if Ka≫1 then Kb<Kw which is weaker than water
- Hence, strong acids and bases do not have conjugates, rather they produce spectator ions which are not acid or basic but neutral.
A Simple Rule
- Linus Pauling developed a rule for strong acids
- For neutral oxyacids, they can be classified as strong is the number of oxygen atoms exceeds the number of hydrogen atoms by two or more
- Strong: HClO4 and HClO3
- Weak: HClO2 and HClO
- For weak acids, the relative strength depends on the difference in oxygen-hydrogen numbers and on the electronegativity of the central atom
Polyprotic Acids
- Acids that can donate more than one proton are called polyprotic - H2SO4.
- While the first proton might come off easily, it get harder and harder for subsequent protons
- In fact, the pKa of the sequential protons are separated by about 5 pH units
- For phosphoric acid, the pKa's are 2.15, 7.20, and 12.35.
Amphoteric Compounds
- Amphoteric substance are those compounds that can act as either and acid or a base (e.g. water)
- When an acid is dissociating in water, the water is acting as a base
- When a base is dissociating in water, the water is acting as an acid
- The strongest acid we can make in water is H+(aq) and the strongest base we can make in water is OH−(aq).
Solvent Leveling
- Solvent leveling occurs when a strong acid is placed in a solvent
- Because the strong acid donates its proton to the solvent. the strongest possible acid is the conjugate acid of the solvent
- This means that the strength of acids such as HCl and HBr cannot be differentiated in water
- To determine the strengths of these acids, we must typically work in non-aqueous solvents
Nonaqueous Solutions
- The Brønsted theory encompases any type of solventthat can donate and accept H+
- The strength of an acid or base varies depending on the solvent
- For instance liquid ammonia NH3(l)+NH3(l)⇌NH+4(l)+NH−2(l) K≈10−30
- Because ammonia is a basic solvent, it enhanced the acidity and suppresses the basicity of substances dissolved in it
- Strong acids that are levelled in water have different acid strengths in acidic solvents such as HF or anhydrous acetic acid
Acidity in Nonaqueous Solvents
- It is difficult to quantify precisely the acidity and basicity in nonaqueous solvents
- One good relative measure is the Hammett acidity function, H0 H0=pKa+log([base][conjugate acid])
- For non-aqueous solvents, or for acidic or basic compounds dissolved in solvents that do not themselves dissociate, H0 is a rough measure of the pH of the solvent or compound in question
- Anhydrous HF and H2SO4 have H0 value of approximately −10 and −12 respectively
Superacids and Superbases
- The term superacid was originally coined by James Bryant Conant in 1927 to describe acids that were stronger than conventional mineral acids
- George A. Olah prepared the so-called magic acid, so-named for its ability to attack hydrocarbons, by mixing antimony pentafloride (SbF5) and fluorosulfonic acid (FSO3H)
- The name was coined after a candle was placed in the mixture and the candle dissolved showing it had been protonated.
Examples of Superacids and Superbases
- Superacids are useful in reactions such as the isomerization of alkanes
- Industrially, anhydrous acid-exchanged zeolites, which are superacid catalysts, are used on a massive scale to isomerize hydrocarbons in the processing of crude oil into gasoline
- Superbases such as lithium diethylamide (LiNEt2), alkyllithium compounds (RLi), and Grignard reagents (RMgX) are usefulin a broad range of organic reactions
- LiNEt2 deprotonates C−H bonds to generate reactive carbanions. RLi and RMgX are powerful nucleophiles
Carbon Acid Acidities in pKa in DMSO
| name | formula | pKa |
|---|---|---|
| Methane | CH4 | ∼56 |
| Propene | C3H6 | ∼44 |
| Benzene | C6H6 | ∼43 |
| Acetylene | C2H2 | 25 |
| Cyclopentadiene | C5H6 | 18 |
Acid-Base Equilibria in Molten Salts
- When a solid salt melts, it forms a solution of the cations and anions
- For example KOH melts at a temperature of 400circC and dissociates into K+ and OH− ions
- These ions can act as a solvent for chemical reactions
- Because of the autodissociation of the OH− ion, water is always present in a molten KOH flux
- Therefore, water is the stronges acid that can exist
- The conjugate base of the solvent, O2−, is the strongest base
- Therefore, a "wet" flux is more acidic while a "dry" flux is more basic
- Mixture of NaOH and KOH are relatively low melting (≈200∘C) and can be used as solvents for crystallizing a variety of basic oxides
The Lewis Theory of Acids and Bases
- A Lewis acid is an electron pair acceptor.
- A Lewis base is an electron pair donor.
- The Lewis classification of acids an bases is broader than the Brønsted-Lowry definition
- It encompasses many more substances
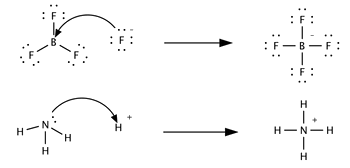
Example 4.1
Identify the Lewis Acid and Lewis Base in the following examples
- I2+I−⇌I−3
- AuCl3+Cl−⇌[AuCl4]−
- Fe3++6H2O⇌[Fe(H2O)6]3+
- TiF4+2F−⇌[TiF6]2−
- SF4+SbF5⇌"SSbF9"
Determining the Strength of Metal Ion Lewis Acids
There are three determing factors in the Lewis acid strength of a metal ion:
- The higher positive charge on the metal, the more acidic it is
- The smaller the atomic radius of the metal ion, the more acidic it is
- For transition metal ions, more electronegative metals tend to make stronger Lewis acids
- Molecules with five coordinte geometries are typically strong Lewis acids because when they accept another pair of electrons they form an octahedral molecule or ion
Lewis Bases Stabilize High Oxidation States
- It turns out that acids tend to stabilize low oxidation states while bases tend to stabilize high oxidation states
- Mn is stable in the +4 oxidation state in K2MnF6 where it is surrounded by six basic F− anions
- The highest stable neutral fluoride of Mn is MnF3
- MnF4 spontaneously decomposes to generate fluorine
Oxide is a Better Base than Fluoride
- Mn can lose all its valence electrons to form Mn7+ in the permanganate ion, MnO−4
- The 7+ oxidation state is stabilized by coordination to four O2− ions
- Because of its 2- charge, O2− is a stronger base and better ion for stabilizing high oxidation states than F−
- The highest oxidation states in the transition metals is usually reached with oxide not fluoride even though fluorine is more electronegative
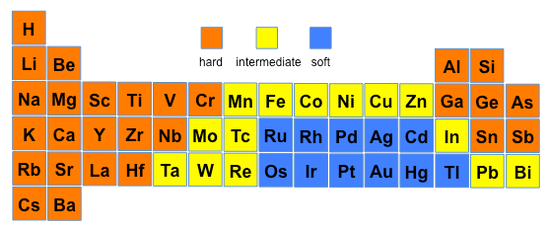
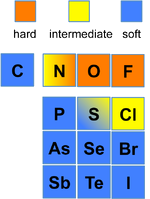
Hard and Soft Acids and Bases
The "hard and soft (Lewis) acids and bases" (HSAB) theory is widely used for explaining stability of compounds, reaction mechanisms, and reaction pathways
Hard Acids and Bases
- "Hard" acids and bases have a high charge (positive for acids, negative for bases) to ionic radius ratio along with higher oxidation states
- Hard acids interact with bases primarily through ionic bonding and they are not very polarizable
- Ex. H+, O2−, OH−, F−, Fe3+, and Al3+
Soft Acids and Bases
- "Soft" acids and bases have a low charg to radius ratio, with low oxidation states
- They are normally larger ions that are polarizable
- Soft acids and bases have significant covalent bonding component when they interact
- Examples: I−, S2−, Ag+
- Some acids and bases are classified as intermediate (neither hard nor soft) such as trimethylborane, Fe2+, and Pb2+
Hard Soft Trends for Acids (Left) and Bases (Right)
Like Binds with Like
- Hards acids iteract more strongly with hard bases than with soft and vice-versa
- Thus, the most stable complexes are hard-hard and soft-soft interactions
| Acid Classification Based on logK1 of the Compound | |||||
|---|---|---|---|---|---|
| fluoride | chloride | bromide | iodide | classification | |
| Fe3+ | 6.0 | 1.4 | 0.5 | - | Hard |
| Pb2+ | 1.3 | 0.9 | 1.1 | 1.3 | Intermediate |
| Ag+ | 0.4 | 3.3 | 4.7 | 6.6 | Soft |
| Hg2+ | 1.0 | 6.7 | 8.9 | 12.9 | Soft |
Gold - The Softest Metal Ion
- Au+ is the softest metal ion
- It forms stable complexes with phosphines and CN−, but not with hard bases such as O2− or F−
- The affinity of Au+ for the soft base CN− is high
- The resulting [Au(CN)2]− complex is so stable that gold can be oxidized by oxygen in the air: 4Au(s)+8CN−(aq)+O2(g)+2H2O(l)→4[Au(CN)2]−(aq)+4OH−(aq)
- The Au3+ is a harder acid and can form complexes with harder bases such as H2O and amines
- AuI (soft-soft) is stable but AuI3 (hard-soft) is unknown
- AuF has never been isolated but AuF3 (hard-hard) is stable.
The ECW Model
- Classification of Lewis acids and bases as hard and soft is useful qualitative approach to rationalize their behavior
- The ECW Model is a more quantitative model that describes and predicts the strength of Lewis acid - Lewis base interactions
- The strength of the interaction is measured as the enthalpy of adduct formation, ΔH −ΔH=EAEB+CACB+W where E is the electrostatic contribution, C is the covalent contribution, and W is the constant energy for cleavage of a dimeric acid or base.
Example of W
- For \([Rh(CO)_2Cl]_2, determine cleavage of the dimer 12[Rh(CO)2Cl]2W=−10.39kcalmol
- For the H-bonding acid (CF3)3COH, W is the enthalpy needed to cleave the internal hydrogen bonding
C5H5N with bis(hexafloroacetyacetonato)copper (II) (Cu(HFacac)2) Example
- In this case, W=0 since neither the acid nor the base is a dimer
- The E and C parameters can be found in various places include the Wikipedia page of the ECW Model
- EA=1.82 and EB=1.78
- CA=2.86 and CB=3.54
- Now we can calculate our value −ΔH=EAEB+CACB+W=(1.82)(1.78)+(2.86)(3.54)=13.4kcalmol
- So, ΔH=−13.4kcalmol and the experimental value for this is −13.4kcalmol.
Comparison with Experiment
- While in our previous example, the calculated and the experimental values were the same, that is not always the case
- The calculated value for Me3B with Me3N is larger than experimental
- These discrepancies ae attributed to the amount of steric effect in the actual system - steric effect between the methyl group in Me3B and Me3N
- When π bonding contributes to the measured enthalpy, the enthalpy calculaed from E and C will be less than the measured value
- The difference gives a measure of the extent of the π bonding contribution
1 / 32