Foundations of Quantum Mechanics
Shaun Williams, PhD
Prelude to the Foundations of Quantum Mechanics
- Quantum mechanics were invented to explain experimental observations
- Things began in 1900 when Max Planck introduced quantization
- In 1925 Erwin Schrödinger and Werner Heisenberg independently introduced two mathematically different but equivalent formulations of a general quantum mechanical theory
- Heisenberg's method uses properties of matrices
- Schrödinger's method uses partial differential equations
- We will develop and utilize Schrödinger's approach
Experimental Observations
- Heisenberg and Schrödinger were inspired by four key experimental observations:
- the spectral distribution of black-body radiation
- the characteristics of the photoelectric effect
- the Compton effect
- the luminescence spectrum of the hydrogen atom
- The explanation of these phenomena required the introduction of two revolutionary concepts:
- physical quantities previously thought to be continuously variable (such as energy and momentum) are quantized
- momentum, \(p\), and wavelength, \(\lambda\), are related, \(p=\frac{h}{\lambda}\), where \(h\) is a fundamental constant
What is Quantized?
- By "quantized" we mean that only certain values are possible or allowed
- Example: Money is quantized since it must be in integer units of the smallest monetary amount, the penny
- It will turn out that many variable we consider to be continuous are actually quantized at the molecular scale.
The Weirdness of Light
- From high school and college physics we know that particles have momentum, \(p=mv\)
- The wave properties of light are clearly demonstrated by interference, diffraction, and refraction effects
- The fact that there is a relationship between momentum (a particle property) and wavelength (a wave property) is weird and it applies to both particles and light
- This was a revolutionary idea
- This relationship is called "wave-particle duality"
- Ultimately, wave-particle duality means that particles have wave-like properties and light waves have particle-like properties.
Black-Body Radiation
- Black-body radiation was one experimental phenomenon that classical physics could not explain
- Example: Hot objects emit EM radiation - the burners on most electric stoves glow red at their highest settings
- If we take a piece of metal and heat it in a flame, it begins to glow, dark red at first, then perhaps white or even blue if the temperature is high enough
- A very hot object would emit a significant amount of energy in the ultraviolet region on the spectrum
- A black-body is an ideal object that emits all frequencies of radiation with a spectral distribution that depends only on the temperature and not on its composition - this is black-body radiation
Explanation of Black-Body Radiation
- It was found that the observed intensity of black-body radiation as a function of wavelength varies with temperature
- Attempts to explain or calculate the spectral distribution from classical theory were all complete failures
- A theory developed by Rayleigh and Jeans predicted that the intensity should go to infinity at short wavelengths
- Since the intensity actually drops to zero at short wavelengths, the Rayleigh-Jean result was called the "ultraviolet catastrophe"
Black-Body Distributions
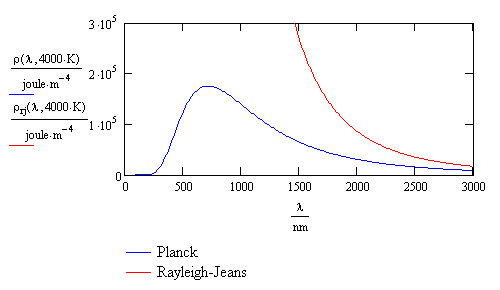
- The Planck and experimental black-body radiation distribution are given in blue
- The Rayleigh-Jean radiation prediction is given in red
- Each curve is for an ideal black-body source at \(4000\, K\)
Planck's Explanation
- Max Planck was the first to successfully explain the spectral distribution of black-body radiation
- Planck said that radiation resulted from oscillations of electrons
- Similarly, oscillations of electrons in an antenna produce radio waves
- Planck realized that in order to explain the spectral distribution, he needed to assume that the energy, \(E\), of the oscillating electrons was quantized and proportional to integer multiples of the frequency \(\nu\) $$ E=nh\nu $$ where \(n\) is an integer and \(h\) is a proportionality constant
Planck's Equation
- Planck was able to derive an equation that gave excellent agreement with the experimental observations for all temperature as long as \(h=6.62618\times 10^{-34}J\cdot s\)
- This fundamental constant, which is an essential component of quantum mechanics, is now called Planck's constant
- The Boltzmann constant, \(k_B\), and the speed of light, \(c\), also appear in the equation $$ \begin{equation} \rho\left( \lambda , T \right) d\lambda = \frac{8\pi hc}{\lambda^5}\frac{d\lambda}{e^{\frac{hc}{\lambda k_B T}}-1} \label{eq:Planck} \end{equation} $$
- In this equation, \( \rho \left( \lambda , T \right) d\lambda \), the radiation density between \(\lambda\) and \( \lambda + d\lambda\) inside the cavity from which the black-body radiation is emitted.
- Coming up we will see that the energy carried by light also is quantized in units of \(h\widetilde{\nu}\) - packets of energy called "photons".
Example 2.1
Use equation \eqref{eq:Planck} to show that the units of \( \rho \left( \lambda, T\right) d\lambda \) are \( \frac{J}{m^3}\) as expected for an energy density.
Example 2.2
Use equation \eqref{eq:Planck} to prepare computer-generated graphs showing how \( \rho \left( \lambda , T\right) \), which is the black-body radiation density per \(nm\), varies with wavelength at various temperatures. Use these graphs to explain why white hot is hotter than red hot.
Example 2.3
Use the results from Example 2.2.2 to prepare a computer-generated graph of \( \lambda_{max}\), which is the peak (or maximum) of the functions plotted in Example 2.2.1, as a function of \(T\). Describe how the color of the light emitted from the black-body varies with temperature.
Example 2.4
Use the results from Example 2.2.4 to estimate the color temperature of sunlight (that has a maximum at \(480\, nm\)) and the temperature of a tungsten light bulb (that has a maximum at \(1035\, nm\).)
Photoelectric Effect
- In the photoelectric effect, light incident on the surface of a metal causes electrons to be ejected
- The number of emitted electrons and their kinetic energy can be measured as a function of the intensity and frequency of light
- Everyone thought that the energy of the light wave (its intensity) should be transfered to the kinetic energy of the emitted electrons
- It was also assumed that the number of electrons that break away from the metal should change with frequency of the light wave
- The classical expectation of the photoelectric effect was that the number of emitted electrons would depend upon the frequency, and their kinetic energy should depend upon the intensity of the light wave
Observation of the Photoelectric Effect
- The actual behavior of the photoelectric effect was completely the opposite of what was expected
- The intensity affects the number of electrons
- The frequency affects the kinetic energy of the emitted electrons
Plots of the Photoelectric Effect
- The kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity
- The number of electrons emitted per second is independent of frequency and increases linearly with the light intensity.
Albert Einstein
- In 1905, Albert Einstein explained the observations of the photoelectric effect
- His hypothesis was that energy carried by light exists in packets of an amount \(h\nu\)
- Each packet, or photon, could cause one electron to be ejected
- The number of electrons ejected therefore depends upon the number of photons, ie. the intensity of the light
- Some of the energy in the photon is used to overcome the binding energy of the electron in the metal
- This binding energy is called the work function, \(\Phi\)
- The remaining energy appears as the kinetic energy, \(\frac{1}{2}mv^2\), of the emitted electron
Einstein's Photoelectric Effect Equations
$$ \begin{align*} E_{photon} &= K_{electron} + W_{electron} \\ h\nu &= \frac{1}{2}mv^2 + \Phi \end{align*} $$ Rearranging this equation reveals the linear dependence of kinetic energy on frequency as seen in the previous plots $$ \frac{1}{2}mv^2 = h\nu - \Phi $$
- The slope of the straight line obtained by plotting the kinetic energy as a function of frequency above the threshold frequency is just Planck's constant
- The x-intercept, where \(\frac{1}{2}mv^2=0\), is just the work function of the metal, \(\Phi = h\nu_0\)
Example 2.5
Sodium metal has a threshold frequency of \(4.40 \times 10^{14}\, Hz\). What is the kinetic energy of a photoelectron ejected from the surface of a piece of sodium when the ejecting photon is \(6.20 \times 10^{14}\, Hz\)? What is the velocity of this photoelectron? From which region of the electromagnetic spectrum is this photon?
The Compton Effect
- The Compton effect concerns the inelastic scattering of x-rays by electrons
- Scattering means dispersing in different directions
- Inelastic means that energy is lost by the scattered object in the process
- The intensity of the scattered x-ray is measured as a function of the wavelength shift \(\Delta \lambda\), where $$ \lambda' = \lambda + \Delta \lambda $$ and the scattering angle is \(\theta\)
The Compton Effect Diagram
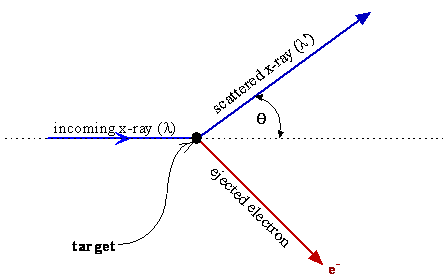
- The x-ray scatters and causes an electron with mass \(m_e\) to be ejected from the object with a direction that conserves the momentum of the system
- Momentum and energy conservation equations then explain the scattering angles and the observed wavelength shift of the x-ray (momentum is taken to equal \(\frac{h}{\lambda}\) and the energy is \(h\nu\))
The Compton Effect Equations
- The situation and basic equations described on the previous slide lead to an equation governing this effect $$ \Delta \lambda = \frac{h}{m_e c}\left( 1 - \cos \theta \right) $$
- This equation describes the experimental data for the variation of \(\Delta \lambda\) with \(\theta\)
- The success of using energy and momentum conservation for two colliding particles to explain the experimental data for the Compton effect is powerful evidence that EM radiation has momentum just like a particle
- The momentum and energy of EM radiation is \(\frac{h}{\lambda}\) and \(h\nu\) respectively
Example 2.6
For Compton scattering, determine the wavelength shift at a scattering angle of \(90^\circ\), and identify the scattering angles where the wavelength shift is the smallest and the largest
Hydrogen Luminescence
- The luminescence spectrum of the hydrogen atom reveals light being emitted at discrete frequencies (line spectra)
- These lines, occurring in groups, are found in different regions of the spectrum: some visible, some infrared, and some in the vacuum ultraviolet
- Spectroscopists looked for a regularity or pattern in the lines
The Rydberg Equation
- Johannes Rydberg recognized a pattern and expressed it in terms of the formula $$ \begin{equation} \widetilde{\nu} = R_H \left( \frac{1}{f^2} - \frac{1}{i^2} \right) \label{eq:Rydberg} \end{equation} $$
- In the equation, \(\widetilde{\nu}\) is the "frequency" of the line in wavenumbers $$ \widetilde{\nu} = \frac{\nu}{c} $$
- \(R_H\) (now called the Rydberg constant) is \( 109677.581\, cm^{-1} \)
- \(f\) and \(i\) are positive integers with \( i \gt f \)
- Different groups of lines, called Rydberg series, are obtained for different values of \(f\), while each line in the series is a different value of \( i\)
Example 2.7
Calculate the wavelength of a line in the hydrogen atom luminescence spectrum corresponding to \(f=7\) and \(i=8\). In which region of the electromagnetic spectrum will this line appear?
What is Behind the Rydberg Equation
- Since the Rydberg equation was derived empirically (ie. invented to describe experimental data), a reason for it was needed
- In others word, scientists now needed a physical origin for the integer values of \(f\) and \(i\) from theory
- This was enormously difficult due to the existing physical theories at that time
Early Models of the Hydrogen Atom
- Ernest Rutherford has proposed a model of atoms based on the \(\alpha\)-particle experiments of Hans Geiger and Ernest Marsden
- The experiment showed that the heavy particles (the nuclei) must occupy a very small region in the atom and that most of the space must be empty or occupied by very low-mass particles (the electrons)
- Problem: The Coulomb force between oppositely charged particles means that the atom should collapse
- Hypothesis: The electrons orbit the nucleus
- New problem: Because the electron would be angularly accelerating it should radiate energy and then its orbit would decay and the atom would collapse again
Neils Bohr's Approach
- In 1913, Niels Bohr proposed that
- The electron could orbit the nucleus in a stationary state without collapsing
- These orbits have discrete energies and radiation is emitted at a discrete frequency when a electron makes a transition between orbits
- The energy difference between the orbits is proportional to the frequency of radiation emitted $$ E_f - E_i = \Delta E_{fi} = h\nu $$
- The angular momentum, \(M\), of the orbiting electron is a positive integer multiple of \(\frac{h}{2\pi}\), which is written as \(\hbar\) and called h-bar $$ M=n\hbar $$ where \(n=1,2,3,\dots\)
Derivation of the Rydberg Equation from Bohr's Model
- Bohr postulated that electrons existed in orbits or states that had discrete energies
- We want to calculate the energy of these states and then take the differences to obtain the energy that is released as light
- Because the proton is so much more massive than the electron, we can consider the proton to be fixed and the electron rotating around it
- Two particles rotate about the center of mass and the rotation can be described as the rotation of a single particle with a reduced mass
Beginning the Derivation
- We write the energy, \(E\), of an orbit or state as the sum of the kinetic energy, \(T\), and the potential energy, \(V\), of the rotating electron
- The potential energy is just the Coulomb energy for two particles with charges \(q_1\) and \(q_2\)
- Therefore $$ \begin{align} E &= T+V \label{eq:3} \\ T &= \frac{1}{2} m_e v^2 \label{eq:4} \\ V &= \frac{q_1 q_2}{4\pi \varepsilon_0 r} = \frac{\left( Ze \right)\left( -e \right)}{4\pi \varepsilon_0 r} = \frac{-Ze^2}{4\pi \varepsilon_0 r} \label{eq:5} \end{align} $$
- \(Ze\) is the nuclear charge, the charge on an electron is \(-e\), \(\varepsilon_0\) is the permittivity of free space (\(8.85419\times 10^{-12}\, C^2 N^{-1} m^{-2}\))
Continuing the Derivation with the Virial Theorem
- We now need to invoke the Virial Theorem for electrostatic forces which comes from an analysis of the forces acting on a system of charged particles
- This theorem says that the total energy of the system is equal to half of its potential energy and also equal to the negative of its kinetic energy $$ \begin{equation} E = \frac{V}{2}=-T \label{eq:6} \end{equation} $$
Remembering Kinetic Energy
- Let's now express the kinetic energy of the electron in terms of its angular momentum $$ T = \frac{1}{2}I\omega^2 $$
- We know that for a sphere at the end of a massless rod of length \(r\), the moment of inertia is \(I=mr^2\) (in our case \(m=m_e\)) so $$ T = \frac{1}{2} m_e r^2 \omega^2 $$
- Angular velocity, \(\omega = \frac{v}{r}\), so $$ T = \frac{1}{2} m_e r^2 \left( \frac{v}{r} \right)^2 = \frac{1}{2} \frac{m_e r^2 v^2}{r^2} $$
Putting Angular Momentum In
- Angular momentum is \(M=m_e vr\)
- To get this into our equation, we need another \(m_e\) on top so we multiply by \(\frac{m_e}{m_e}\) $$ T = \frac{1}{2} \frac{m_e^2 r^2 v^2}{m_e r^2} = \frac{M^2}{2m_e r^2} $$
Putting Some Things Together
- Remember equation \eqref{eq:5}: \( V=\frac{-Ze^2}{4\pi \varepsilon_0 r} \)
- Plugging our expression for \(V\) and \(T\) into equation \eqref{eq:6} we find that $$ \begin{align*} \frac{V}{2} &= -T \\ \frac{1}{2}\frac{-Ze^2}{4\pi \varepsilon_0 r} &= -\frac{M^2}{2m_e r^2} \\ \frac{Ze^2}{4\pi \varepsilon_0 r} &= \frac{M^2}{m_e r^2} \\ \end{align*} $$
Solving for the Orbital Radius
- Let's isolate the orbital radius, \(r\) $$ \begin{align*} \frac{m_e r^2 Ze^2}{4\pi \varepsilon_0 r} &= M^2 \\ r &= \frac{M^2 4 \pi \varepsilon_0}{m_e Ze^2} \end{align*} $$
- We can now introduce Bohr's proposal that angular momentum is quantized, \(M=n\hbar\) $$ \begin{equation} r_n = \frac{n^2 \hbar^2 4 \pi \varepsilon_0}{m_e Ze^2} = \frac{4 \pi \varepsilon_0 n^2 \hbar^2}{m_e Ze^2} \label{eq:Bohr} \end{equation} $$ please note that the "4" in the numerator is missing in the textbook
The Bohr Radius
- Notice that the quantization of angular momentum results in the quantization of the radii of the orbits
- The smallest radius, for the orbit with \(n=1\), is called the Bohr radius and is denoted by \(a_0\) $$ a_0 = 52.92\, pm = 0.5292 \AA = 5.292\times 10^{-11}\, m $$
Calculating Total Energy
- From before, our total energy is given as $$ E = \frac{V}{2}=-T $$ so plugging in \( T = \frac{M^2}{2m_e r^2} = \frac{n^2 \hbar^2}{2m_e r_n^2} \) from before $$ E = -\frac{n^2 \hbar^2}{2m_e r_n^2} $$
- Substituting our equation for the quantized radius $$ E_n = -\frac{n^2 \hbar^2}{2m_e \left( \frac{4 \pi \varepsilon_0 n^2 \hbar^2}{m_e Ze^2} \right)^2} $$
Simplifying Our Energy Expression
- Doing some basic algebra $$ \begin{align*} E_n &= -\frac{n^2 \hbar^2}{2m_e} \left( \frac{m_e Ze^2}{4 \pi \varepsilon_0 n^2 \hbar^2} \right)^2 \\ E_n &= -\frac{n^2 \hbar^2 m_e^2 Z^2 e^4}{2m_e 4^2 \pi^2 \varepsilon_0^2 n^4 \hbar^4} \\ E_n &= -\frac{m_e Z^2 e^4}{32 \pi^2 \varepsilon_0^2 n^2 \hbar^2} \\ \end{align*} $$ Remebering that \(\hbar=\frac{h}{2\pi}\) $$ \begin{equation} E_n = -\frac{m_e Z^2 e^4}{32 \pi^2 \varepsilon_0^2 n^2 \left( \frac{h}{2\pi}\right)^2} = -\frac{m_e Z^2 e^4}{8 \varepsilon_0^2 n^2 h^2} \label{eq:8} \end{equation} $$
Example 2.8
Calculate the potential energy, the kinetic energy, and the total energy for hydrogen when \(r=52.92\, pm\).
Example 2.9
Sketch an energy level diagram for the hydrogen atom. Label each energy level with the quantum number \(n\) and the radius of the corresponding orbit.
Example 2.10
Calculate a value for the Bohr radius using our equation to check that this equation is consistent with the value \( 52.9\, pm\). What would the radius be for \( n = 1 \) in the \(\chem{Li^{2+}}\) ion.
Example 2.11
How do the radii of the hydrogen orbits vary with \(n\)? Prepare a graph showing \(r\) as a function of \(n\). To which family of curves does this plot belong? States of hydrogen atoms with \(n = 200\) have been prepared. What is the diameter of the atoms in these states? Identify something else this size.
Explaining the Hydrogen Luminescene Spectrum
- Bohr said that light of frequency \(\nu_{if}\) is produced when an electron goes from an orbit with \(n=i\) (where "i" represents "initial") to a lower energy orbit \(n=f\) (where "f" represents "final"), with \(i \gt f\)
- So $$ \begin{align*} E_{photon} &= h\nu_{if} = E_i-E_f = \Delta E_{if} \\ h\nu_{if} &= -\frac{m_e Z^2 e^4}{8 \varepsilon_0^2 f^2 h^2} - -\frac{m_e Z^2 e^4}{8 \varepsilon_0^2 i^2 h^2} = -\frac{m_e Z^2 e^4}{8 \varepsilon_0^2 h^2} \left( \frac{1}{f^2} - \frac{1}{i^2} \right) \end{align*} $$ Note that the overall minus sign indicates that light (energy) is leaving
- Since \(\nu_{if}=c\widetilde{\nu}_{if}\) $$ \begin{equation} \widetilde{\nu}_{if} = -\frac{m_e Z^2 e^4}{8 \varepsilon_0^2 c h^3} \left( \frac{1}{f^2} - \frac{1}{i^2} \right) \label{eq:9} \end{equation}$$
The Rydberg Constant
- We recognize that this functional form is identical to the Rydberg equation
- This means that we now know where the Rydberg constant (and equation) comes from $$ R_H = \frac{m_e e^4}{8 \varepsilon_0^2 c h^3} $$ note that in this equation, \(Z=1\)
Example 2.12
Calculate the energy of a photon that is produced when an electron in a hydrogen atom goes from an orbit with \( n = 4\) to and orbit with \( n = 1\). What happens to the energy of the photon as the initial value of \(n\) approaches infinity?
Summary of Bohr's Contribution
- Bohr's proposal explained the hydrogen atom spectrum, the origin of the Rydberg formula, and the value of the Rydberg constant
- It demonstrated that the integers in the Rydberg formula come from quantization
- The energy, angular momentum, and the radius of the orbiting electron are all quantized
- This quantization means that the orbits are stable and the electron cannot spiral into the nucleus
Problems with the Bohr Model
- Although Bohr's ideas successfully explained the hydrogen spectrum, it failed when applied to the spectra of all the other atoms
- Another question remained, why is angular momentum quantized in units of \(\hbar\)?
- This question was answered by de Broglie and that answer led Schrödinger to a general postulate that produces the quantization of angular momentum as a consequence
The Wave Properties of Matter
- Compton scattering experiments showed the light exhibited particle-like properties with a momentum of \(p=\frac{h}{\lambda}\)
- In 1924, Louis de Broglie proposed that if light waves exhibited properties of particles, then matter particles should exhibit properties of waves and the equation should be the same $$ \lambda = \frac{h}{p} $$
- Since the wave vector \(k\) is defined as \( k= \frac{2\pi}{\lambda}\), we can rewrite this equation as $$ p = \hbar k $$
Example 2.13
Calculate the de Broglie wavelength for an electron with a kinetic energy of \( 1000\, eV\). Could such electrons be used to obtain diffraction patterns of molecules?
Example 2.14
Calculate the de Broglie wavelength for a fast ball thrown at 100 miles per hour and weighing 4 ounces. Comment on whether the wave properties of baseballs could be observed.
de Broglie's Proposal
- The validity of de Broglie's proposal was confirmed by electron diffraction experiments of G. P. Thomson in 1926 and C. Davisson and L. H. Germer in 1927.
- In these experiments, the researchers found that electrons scattered from atoms in a crystal and that those scattered electrons produced an interference pattern
- de Broglie's proposal an be applied to Bohr's view of the hydrogen atom to show why angular momentum is quantized in units of \(\hbar\)
- If the electron in the hydrogen atom is orbiting the nucleus in a stable orbit, then it should be described by a stable or stationary wave (standing wave)
- Such standing waves have maxima and minima of the wave and nodes at the same position
Explaining the \(\hbar\) Quantization
- To place a standing wave in the shape of a round orbit, the circumference \(2\pi r\) must be an integer multiple of the wavelength $$ 2\pi r = n\lambda $$
- Using the wavelength-momentum relationship \(\lambda=\frac{h}{p}\) $$ \begin{align*} 2\pi r &= n \frac{h}{p} \\ rp &= \frac{n h}{2\pi} = n\hbar \\ M &= n\hbar \end{align*} $$
- By saying the electron has the property of a standing wave, we are led to the quantization in terms of \(\hbar\)
/