Lecture 8
Chemical Bonding
Shaun Williams, PhD
Types of Bonds
- Chemical bond
- A force that holds atoms together in a molecule or compound
- Two types of chemical bonds
- Ionic Bonds
- Covalent Bonds
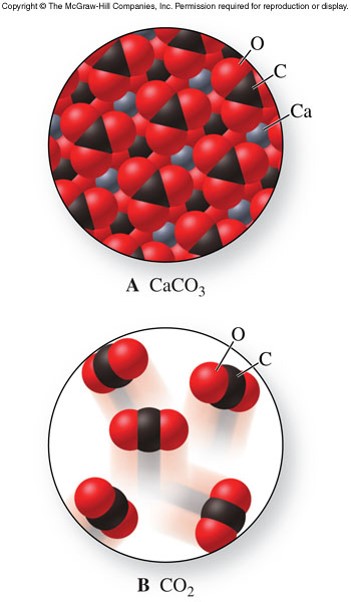
Ionic Bond
- A bond created by electrostatic attraction between oppositely charged ions
- Occurs between a metal and a nonmetal
- Electrons transferred between the cation (positively charged ion) and the anion (negatively charged ion)
- Extremely strong bonds
Covalent Bond
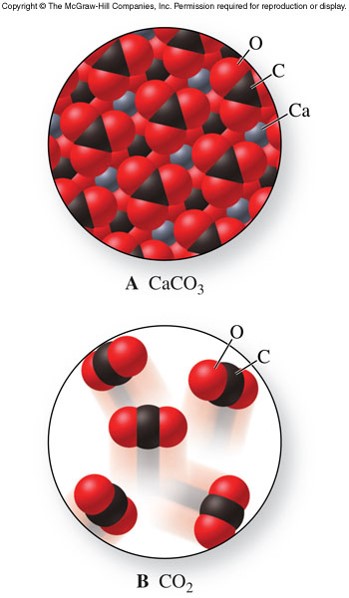
- A bond created by the sharing of electrons between atoms
- Occurs between two nonmetals (resulting in a neutral overall charge)
- Electrons not transferred in this case
- Electrons shared in pairs typically
- Weaker bonds than ionic bonds
Polar vs Nonpolar
- Polar covalent bonds are:
- Typically shorter bonds
- Stronger bonds due to their increased ionic character
- Nonpolar covalent bonds are:
- Typically longer bonds
- Weaker bonds
- Polarity
- Occurs in polar covalent molecules
- Polarity is the degree of transfer of electrons in a covalently bonded molecule composed of different element's atoms.
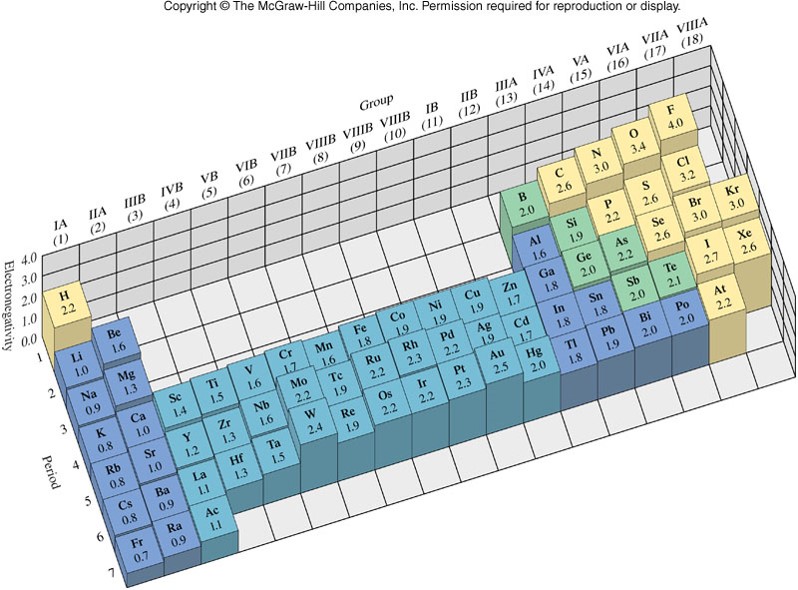
Electronegativity
- Ability of an atom to attract bonding electrons
- Proposed by Linus Pauling in the early 1930's
- A difference in electronegativity between the atoms in a covalent bond results in:
- A polar covalent bond
- Increased ionic character
- The greater the difference in electronegativity, the greater the ionic character and the more polar the bond that joins the atoms.
- Decreased bond length and increased bond strength
- No difference in electronegativity between atoms in a covalent bond results in a nonpolar covalent bond.
Electronegativity Values
Ionic Bonding
- Lewis Dot symbol
- Electron dot symbol
- Dots placed around an element's symbol represent valence electrons
- Pair electrons as needed
- Octet rule
- Tendency of an atom to achieve an electron configuration having 8 valence electrons
- Same as the electron configuration of a noble gas
- The 8 electrons exist in 4 pairs
- Ions achieve 8 electrons by losing or gaining electrons
- H reacts to obtain a total of 2 electrons like He
- Tendency of an atom to achieve an electron configuration having 8 valence electrons
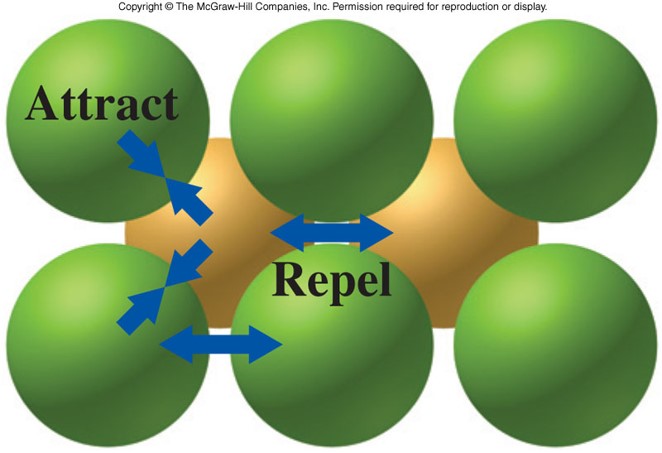
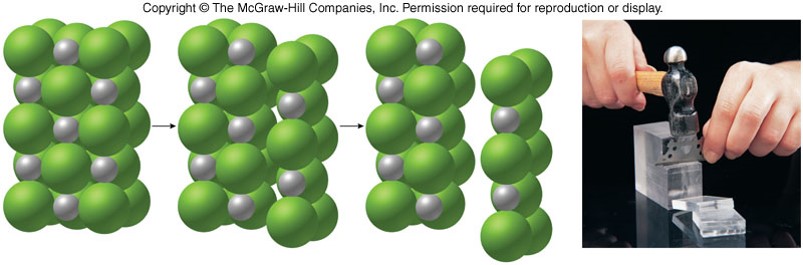
Structures of Ionic Crystals
- Ionic crystal
- Ions are arranged in a regular geometric pattern that maximizes the attractive forces and minimizes the repulsive forces.
- Hard and brittle
- Can shatter if struck forcefully
- The charges and sizes of ions largely determine the characteristic patterns of ionic crystals
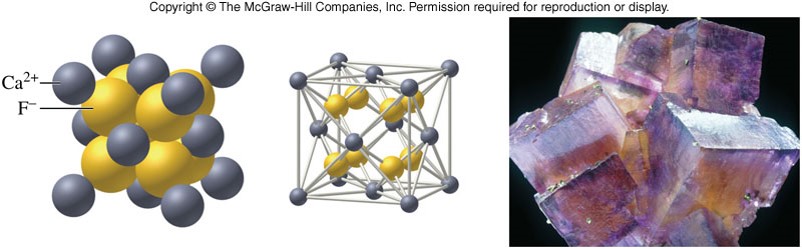
A More Complicated Ionic Crystal
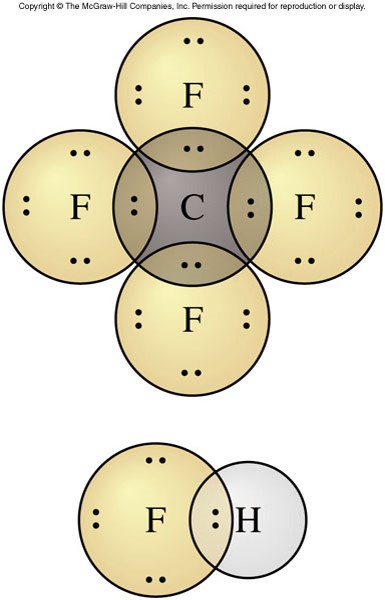
Covalent Bonding
- Single covalent bond
- A covalent bond that consists of a pair of electrons shared by two atoms
- Each atom contributes one electron to the bond
- The orbitals overlap to allow the electron pair to be located around both atoms
- Lewis formula
- The atoms are shown separately and the valence electrons are represented by dots
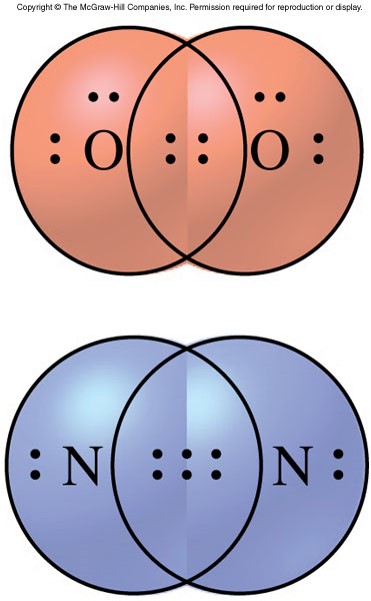
More on Covalent Bonding
- Multiple covalent bonds
- Covalent bonds that consist of more than one pair of electrons shared by two atoms
- Double bond
- Sharing of two pairs of electrons (4 electrons total)
- In Lewis Dot structures, a double bond is represented by 4 dots or 2 parallel lines.
- Triple bond
- Sharing of three pairs of electrons (6 electrons total)
- In Lewis Dot structures, a triple bond is represented by 6 dots or 3 parallel lines.
Steps for Writing Lewis Dot Structures
- Write an atomic skeleton.
- The arrangement of atoms is usually symmetrical.
- In a molecule of two different elements, the one with the greater number of atoms usually surrounds the one with the lesser number of atoms.
- The central atom, the one surrounded by the other atoms, tends to be the one that is less electronegative and is present in the least quantity. This atom usually forms the greater number of bonds and is found further toward the bottom left side of the periodic table.
- Hydrogen atoms are generally on the outside of the molecule.
- The chemical formula may give clues about the arrangement of atoms.
Steps for Writing Lewis Dot Structures (cont.)
- Sum the valence electrons from each atom to get the total number of valence electrons.
- Place two electrons, a single bond, between each pair of bonded atoms.
- If you have not placed all the valence electrons in the formula, add any remaining electrons as unshared electron pairs, consistent with the octet rule.
- Add pairs of electrons first to complete the octet of atoms surrounding the central atom. Then add any remaining electrons in pairs to the central atom.
- If necessary to satisfy the octet rule, shift unshared electrons from non-bonded position on atoms with completed octets to positions between atoms to make double or triple bonds.
Writing Lewis Dot Structures
- Write an atomic skeleton for \(\chem{CH_2O}\):
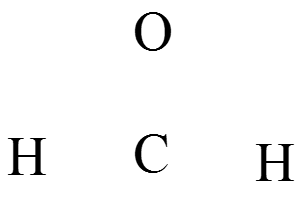
- Sum the valence electrons from each atom to get the total number of valence electrons.
- Carbon is in Group IVA (14), so it has 4 valence electrons.
- Each hydrogen contributes 1 valence electron (H is in Group IA (1)).
- Oxygen contributes 6 valence electrons because it is in Group VIA (16).
- Total number of valence electrons: \( 4 + \left( 1 \times 2 \right) + 6 = 12 \)
Writing Lewis Dot Structures (cont.)
- Next, bond the electrons around each atom in a single bond first, then use double bonds as necessary.
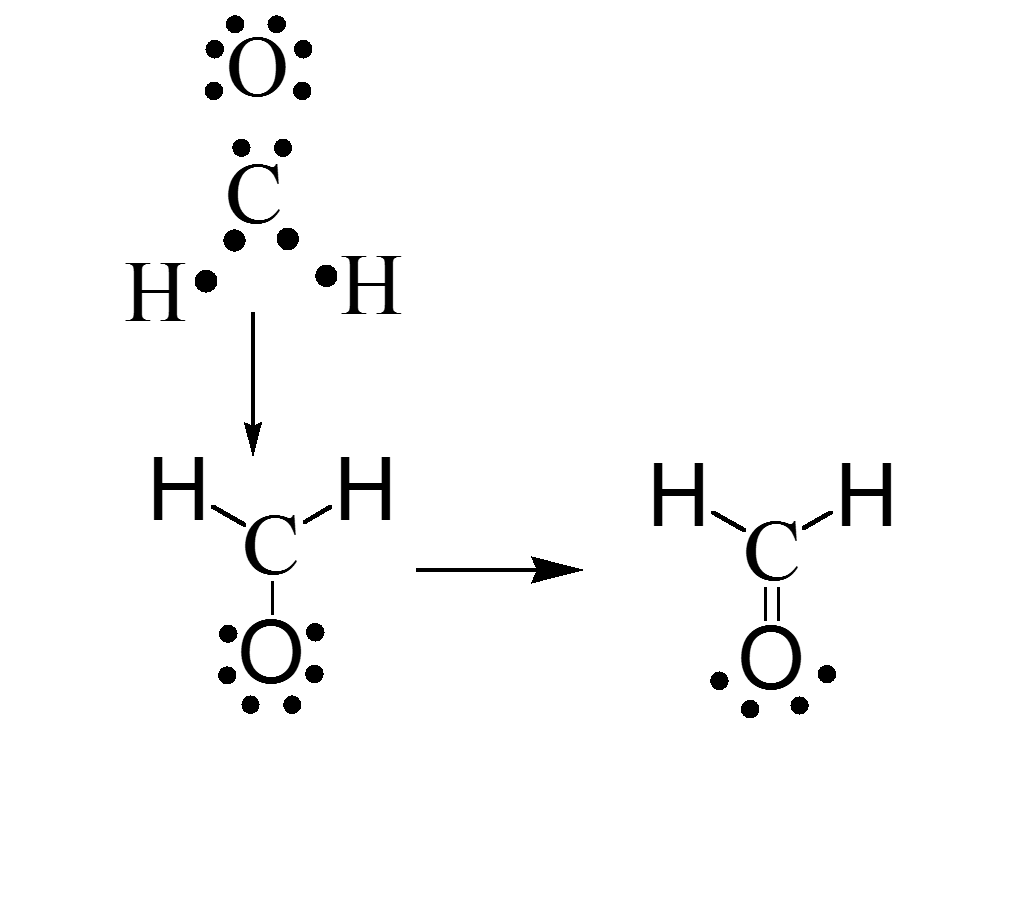
Exceptions to the Octet Rule
- Incomplete octets
- The central atom has less than eight electrons around it.
- Ex. \(\chem{BH_3}\)
- Expanded octets
- The central atom has greater than eight electrons around it.
- Ex. \(\chem{PH_5}\), \(\chem{SF_6}\)
- Odd-numbered Lewis Dot structures
- The total number of electrons is odd.
- The central atom has an odd number of electrons around it.
- Ex. \(\chem{NO}\), \(\chem{NO_2}\), \(\chem{ClO_2}\)
Bonding on Carbon Compounds
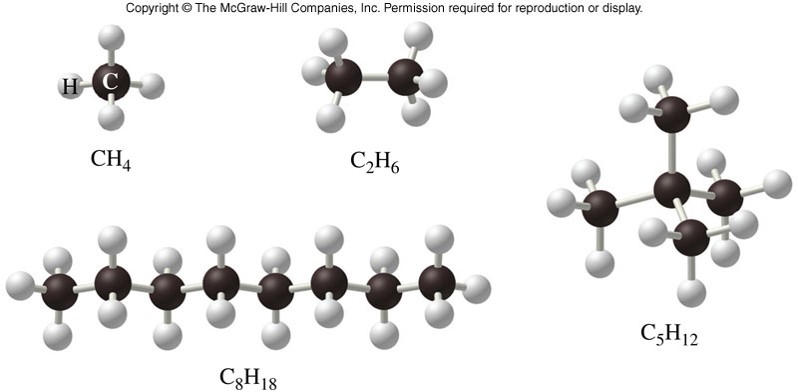
- Carbon has:
- Four valence electrons
- The ability to form four bonds
- The ability to catenate, i.e. bond to itself
- Very strong bonds when bonded to itself
- Carbon molecules are ubiquitous in nature.
Functional Groups
- Functional group
- A group that is introduced into or substituted in a hydrocarbon chain
- Gives the hydrocarbon its characteristic properties
- The group has a heteroatom, an atom other C and H
- Typically O, S, and N
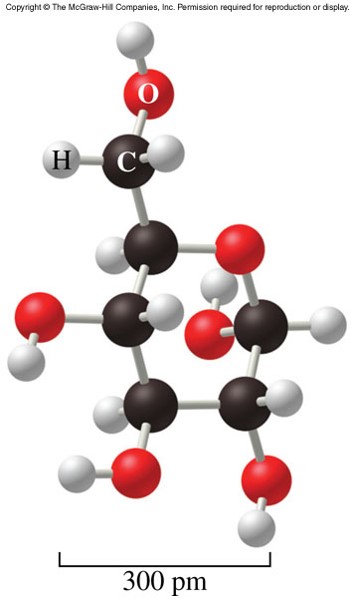
- Alcohol
- A hydroxyl group (-OH) replaces a hydrogen atom in the formula for a hydrocarbon
Shapes of Molecules
- The relative locations of electron pairs around a central atom play a large role in determining a molecule's 3-D shape.
- Negatively charged electrons repel one another, so electron pairs in different orbitals stay as far apart as possible.
How do we determine the shape of molecules?
- Valence shell electron pair repulsion (VSEPR) theory
- The tendency of electron pairs to adjust the orientation of their orbitals to maximize the distance between them
- The bonded atoms and unshared pairs are arranged around the central atom as far apart as possible
- Bond angle
- A shape is characterized by a bond angle between the central atom and the atoms bonded to it
VSEPR Derivative Structures
| General Formula | Number of Bonded Atoms | Number of Lone Pairs | Molecular Shape | Bond Angle | Examples |
|---|---|---|---|---|---|
| \(\chem{AX_2}\) | 2 | 0 | Linear |
\(180^\circ\) | \(\chem{BeCl_2}\), \(\chem{CO_2}\), \(\chem{HCN}\) |
| \(\chem{AX_3}\) | 3 | 0 | Trigonal Planar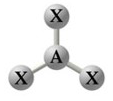 |
\(120^\circ\) | \(\chem{BF_3}\), \(\chem{BH_3}\), \(\chem{SO_3}\), \(\chem{NO_3^-}\) |
| \(\chem{AX_2}\) | 2 | 1 | Bent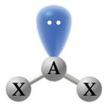 |
\(120^\circ\) | \(\chem{SO_2}\), \(\chem{NO_2^-}\) |
More VSEPR Derivative Structures
| General Formula | Number of Bonded Atoms | Number of Lone Pairs | Molecular Shape | Bond Angle | Examples |
|---|---|---|---|---|---|
| \(\chem{AX_4}\) | 4 | 0 | Teetrahedral |
\(109.5^\circ\) | \(\chem{CH_4}\), \(\chem{CH_2Cl_2}\), \(\chem{SiCl_4}\), \(\chem{POCl_3}\), \(\chem{BrO_4^-}\) |
| \(\chem{AX_3}\) | 3 | 1 | Trigonal Pyramidal |
\(109.5^\circ\) | \(\chem{NH_3}\), \(\chem{PF_3}\), \(\chem{NH_2Cl}\) |
| \(\chem{AX_2}\) | 2 | 2 | Bent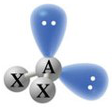 |
\(109.5^\circ\) | \(\chem{H_2O}\), \(\chem{F_2O}\), \(\chem{BrO_2}\), \(\chem{SO_2}\), \(\chem{SCl_2}\) |
Steps for VSEPR Structures
- Draw a Lewis formula.
- Count the number of atoms bonded to the central atom, and count unshared pairs on the central atom.
- Add the number of atoms and the number of unshared electron pairs around the central atom. The total indicates the parent structure.
- The molecular shape is derived from the parent shape by considering only the positions in the structure occupied by bonded atoms.
Polarity of Molecules
- Diatomic molecules
- Polarity lies along the plane of the bond
- Polyatomic molecules
- A nonpolar molecule is one that has all nonpolar bonds or one that has polar bonds that cancel out
- Bonds that cancel out have equal polarities in opposite directions
- This happens when:
- A central atom has no unshared electrons
- The atoms around the central atom all have the same electronegativity
- This happens when:
- Bonds that cancel out have equal polarities in opposite directions
- A polar molecule is one that has polar bonds that DO NOT cancel out
- A nonpolar molecule is one that has all nonpolar bonds or one that has polar bonds that cancel out
"Like" Dissolves "Like"
- Ionic salts and polar liquids dissolve better in polar liquids than in nonpolar liquids
- Nonpolar liquids dissolve better in other nonpolar liquids than in polar liquids

/