Lecture 7
Electron Structure of the Atom
Shaun Williams, PhD
Electromagnetic Radiation and Energy
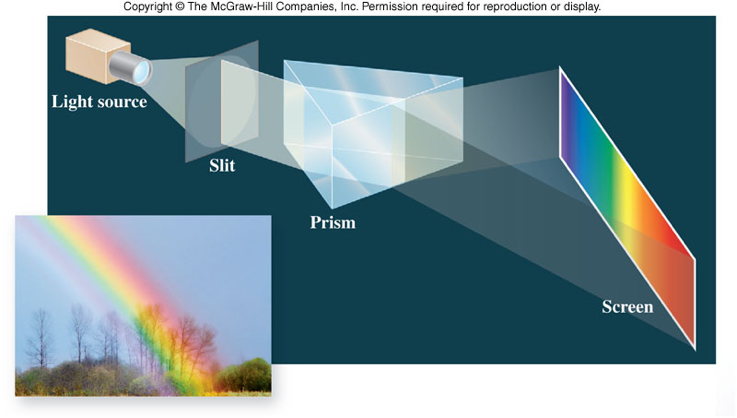
White Light
- Is composed of different colors that can be separated by a prism
- Water acts as a prism for sunlight, giving the effect of a rainbow
- Sources of white light
- Sun
- Regular (incandescent) light bulbs
Electromagnetic Radiation
- A form of energy
- Travels through space at the speed of light (\(3.0 \times 10^8 \bfrac{m}{s}\)) as oscillating waves
- Is both an electric and a magnetic field
- Also called radiant energy
- Some examples of EM radiation
- Light
- X-rays
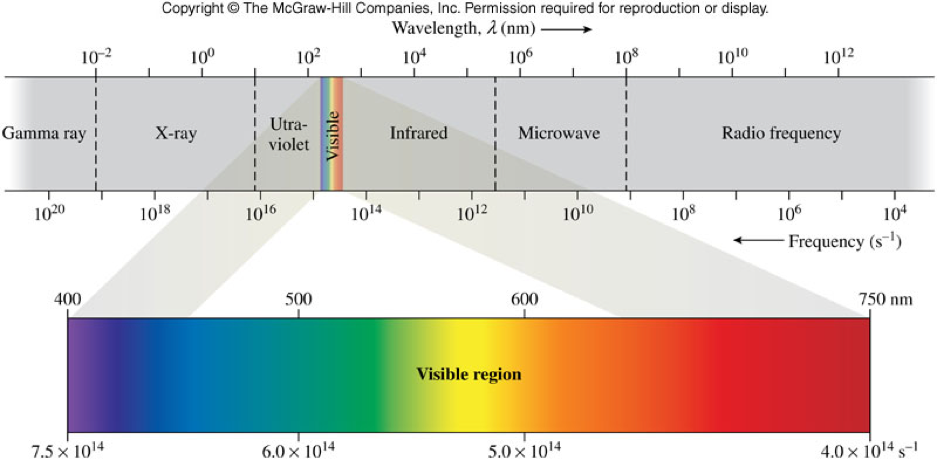
Differentiating the Kinds of Electromagnetic Radiation - Wavelength
- Wavelength (\( \lambda \))
- The distance between two corresponding points on a wave
- Units are same as length - m, or commonly nm (\( 10^{-9}\, \chem{m}\))
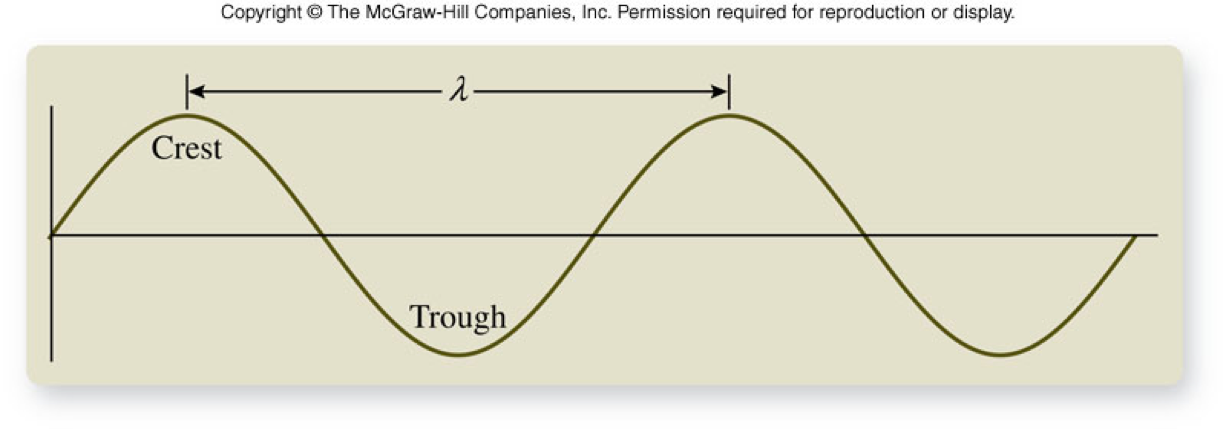
Differentiating the Kinds of Electromagnetic Radiation - Frequency
- Frequency (\(\nu\))
- A measure of the number of wave cycles that move through a point in space in 1 s
- Units are hertz (Hz) which are the same as inverse seconds (1/s)
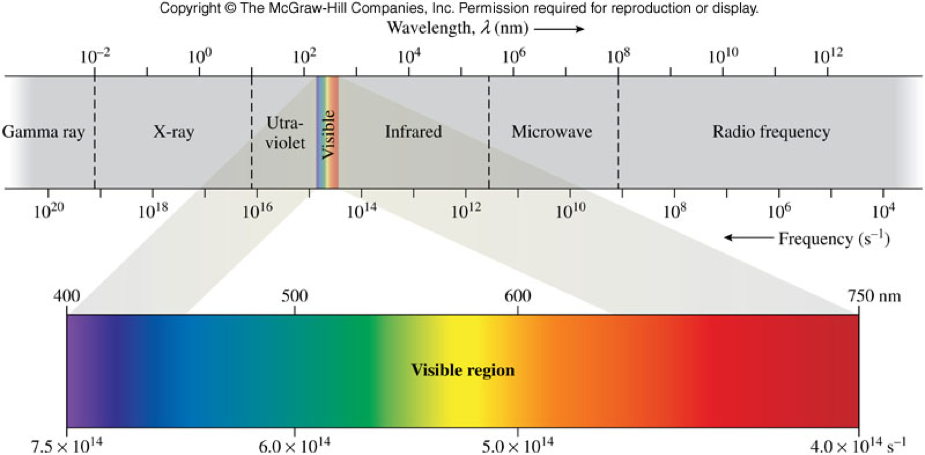
Frequency, Wavelength, and the Electromagnetic Spectrum
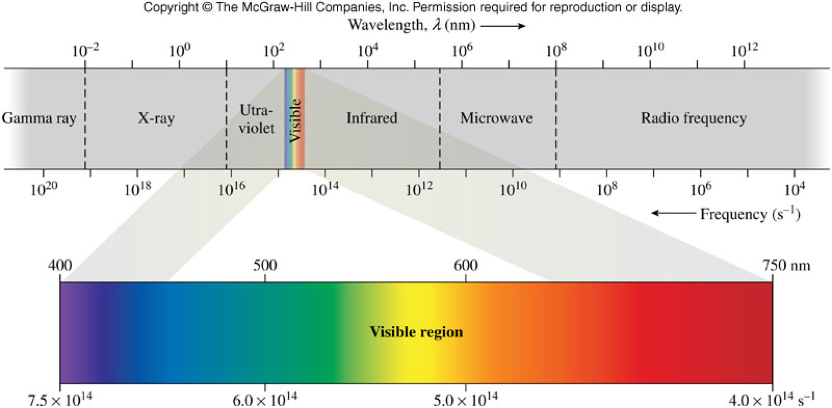
- Frequency and wavelength are inversely proportional
- i.e. as one increases the other decreases \[ c=\lambda \nu \] Where \(c\) = speed of light (\(3.0 \times 10^8 \bfrac{m}{s}\)), \(\lambda\) = wavelength (in meters), and \(\nu\) = frequency (in Hz)
Duality of Light
- Light exists as both waves and particles (or packets of light called photons)
- Characteristics of waves
- Frequency
- Wavelength
- \(c=\lambda \nu\)
- Characteristic of photons
- Energy of a photon
- Is directly proportional to the frequency and inversely proportional to the wavelength \[ E_{photon}=h\nu \] Where \(E_{photon}\) = energy of the photon (in Joules), \(h\) = Planck's constant (\(6.626 \times 10^{-34} Js\)), and \(ν\) = frequency (in Hz)
- Energy of a photon
- Characteristics of waves
\(\lambda\), \(\nu\), and \(\chem{E_{photon}}\)
\[ c=\lambda \nu \;\;\;\;\;\;\; \chem{E_{photon}}=h\nu \]
- Using algebra, we can manipulate these two equations several ways:
- For \(c = \lambda \nu\),
- We can solve for \(\lambda\): \(\lambda = \bfrac{c}{\nu}\)
- or \(\nu\): \(\nu=\bfrac{c}{\lambda}\)
- For \(\chem{E_{photon}} = h\nu\)
- We can substitute \(\bfrac{c}{\lambda}\) for \(\nu\), giving us the equation: \[ \chem{E_{photon}} = \frac{hc}{\lambda} \]
- This equation shows the inverse proportionality between \(\chem{E_{photon}}\) and \(\lambda\) (wavelength)
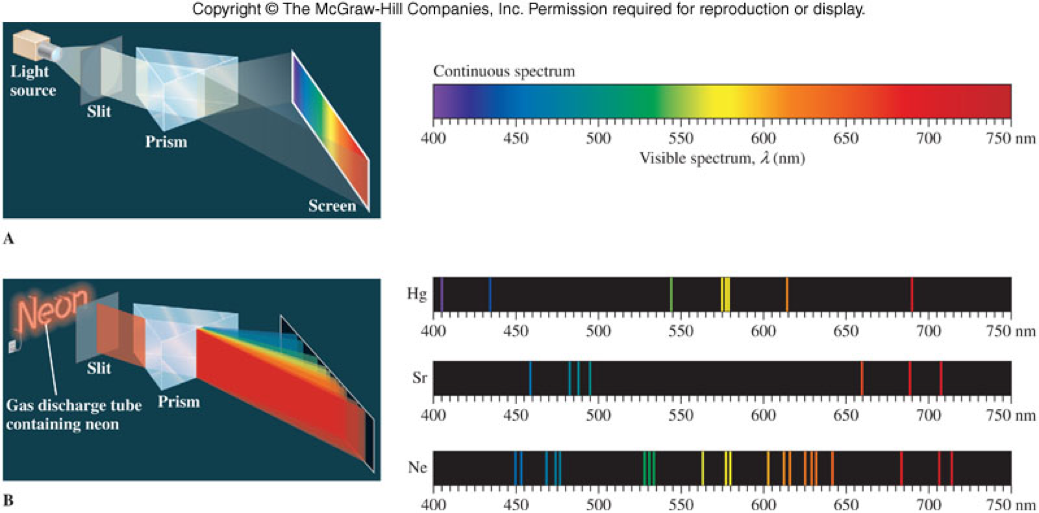
Energy is Quantized!
- Max Planck first hypothesized that energy produced by atoms can only have certain values and is therefore quantized.
- That's the reason why only distinct lines are seen in element line spectras. Energy is quantized and can only exist at certain wavelengths.
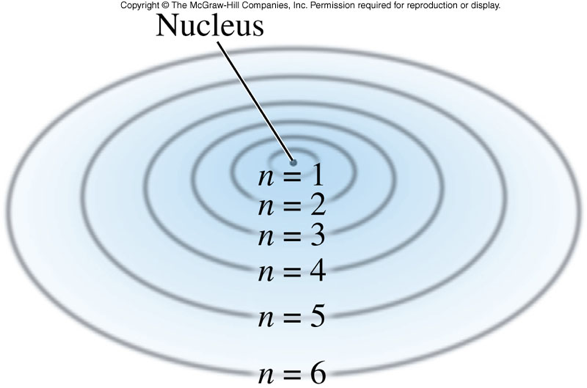
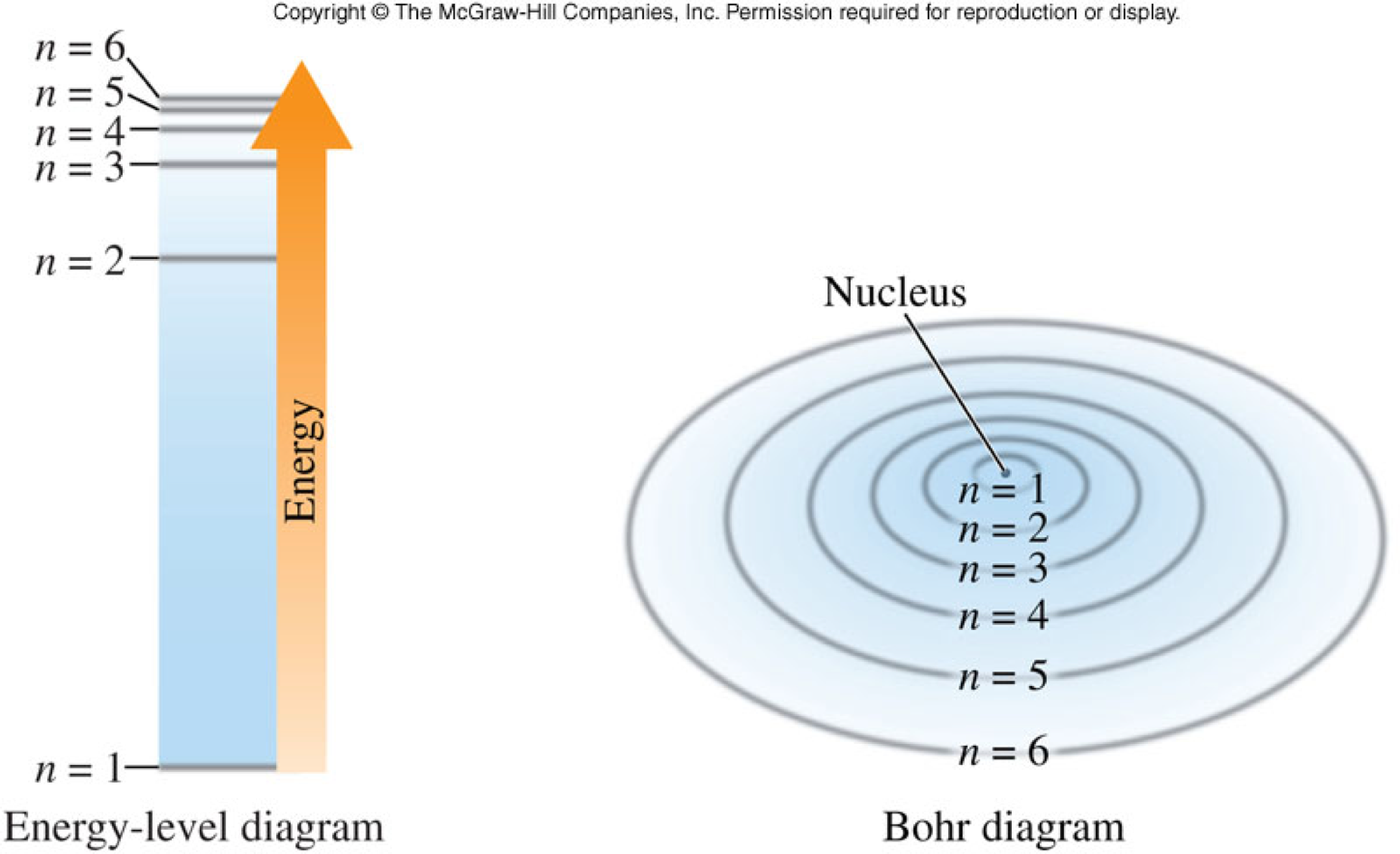
The Bohr Model of the Hydrogen Atom
- Niels Bohr hypothesized that electrons orbit the nucleus just as the planets orbit the sun (planetary model) and have fixed radii.
- He labeled the electron orbits with a number, starting with 1 closest to the nucleus and increasing as the orbits get further away from the nucleus.
- This number is known as the Principal Quantum Number (\(n\)).
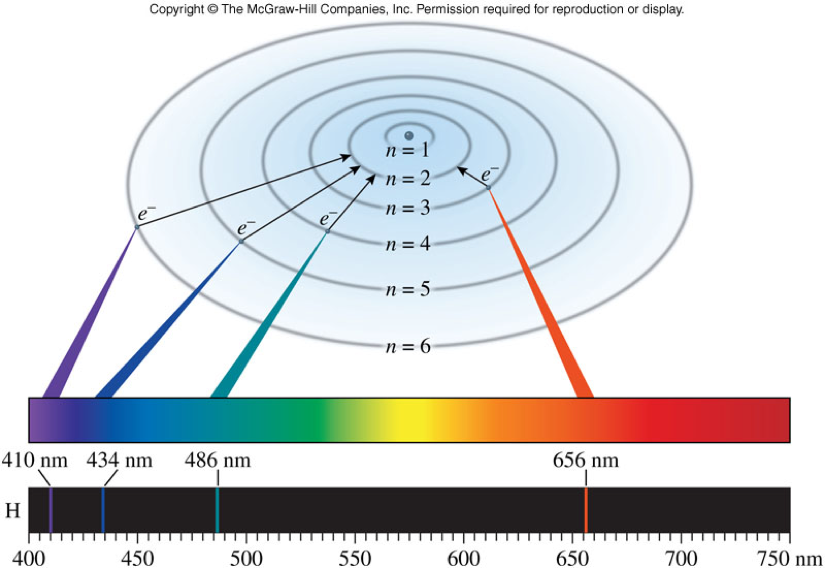
Bohr Model and Light
- The orbit with the lowest energy is closest to the nucleus. The energy of each orbit increases as the orbits get further away from the nucleus.
- When an electron jumps from one orbit to another, it absorbs or emits energy according to the equation: \(\Delta E = E_f – E_i\)
The Modern Model of the Atom
- The modern model of the atom is based on Schrödinger's mathematical model of waves
- This model describes electrons as occupying orbitals, not orbits.
- Orbitals
- Three dimensional regions in space where electrons are likely to be found, not a circular pathway
- Principal energy level
- Orbitals of similar size
- Orbitals
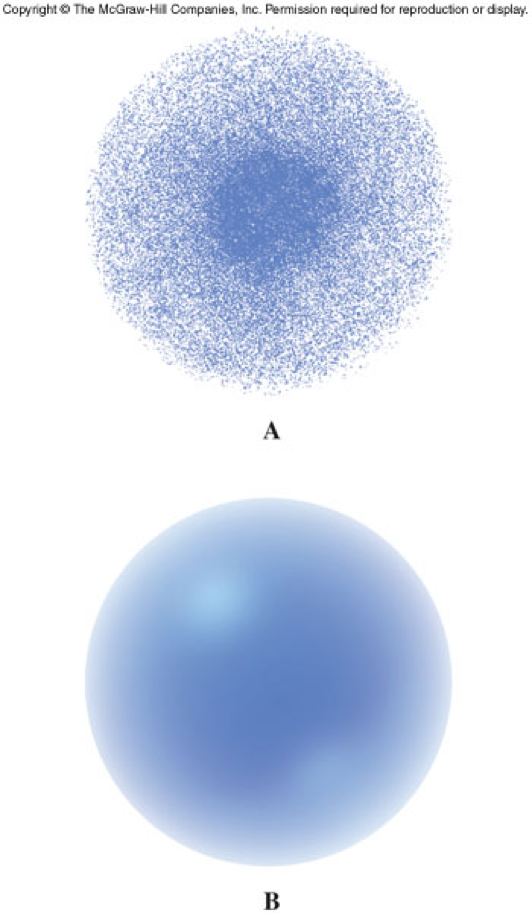
Energy Levels
Orbitals
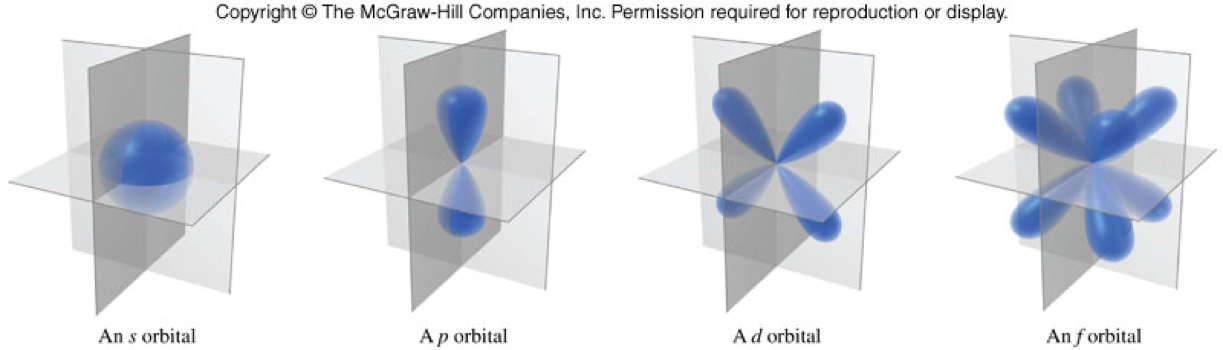
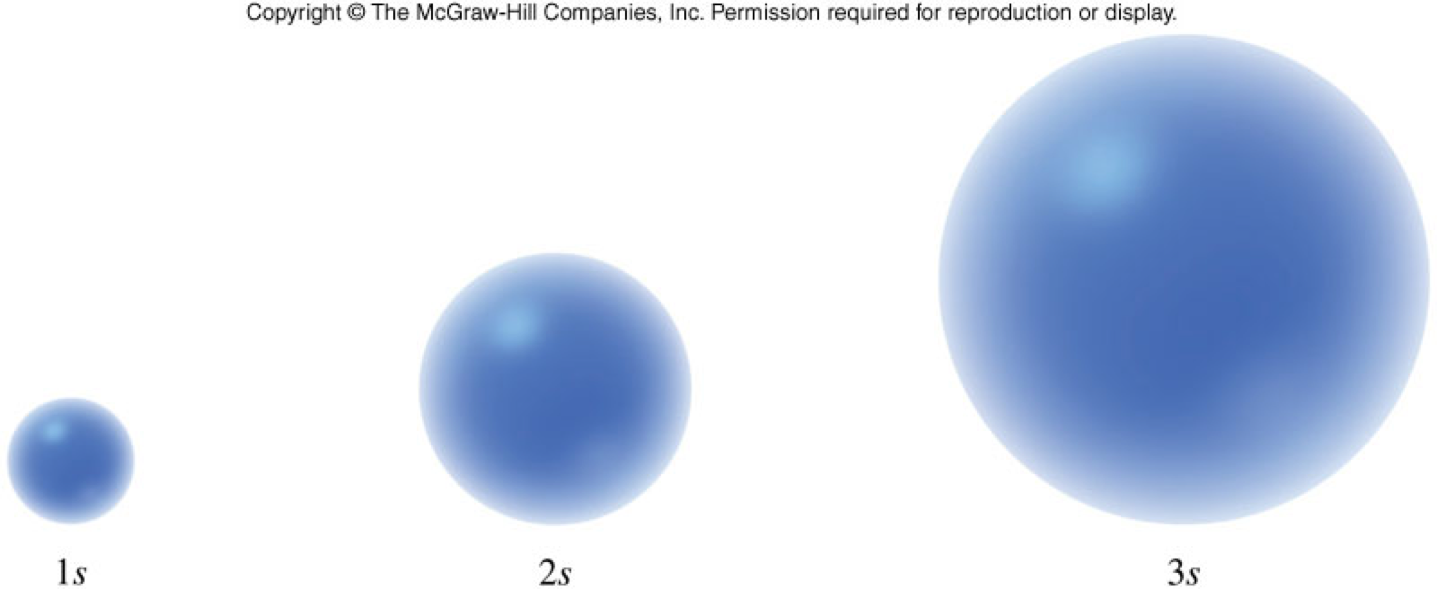
- Come in different shapes and sizes.
- Lower energy orbitals are smaller.
- Higher energy orbitals are larger and extend further away from the nucleus.
- Four most common types are \(s\), \(p\), \(d\), and \(f\).
- Also known as sublevels
- Consists of just one type of orbital at a specific energy level
- The number of sublevels is equal to \(n\), the Principal Quantum Number
\(s\)-Orbitals
\(p\)-Orbitals
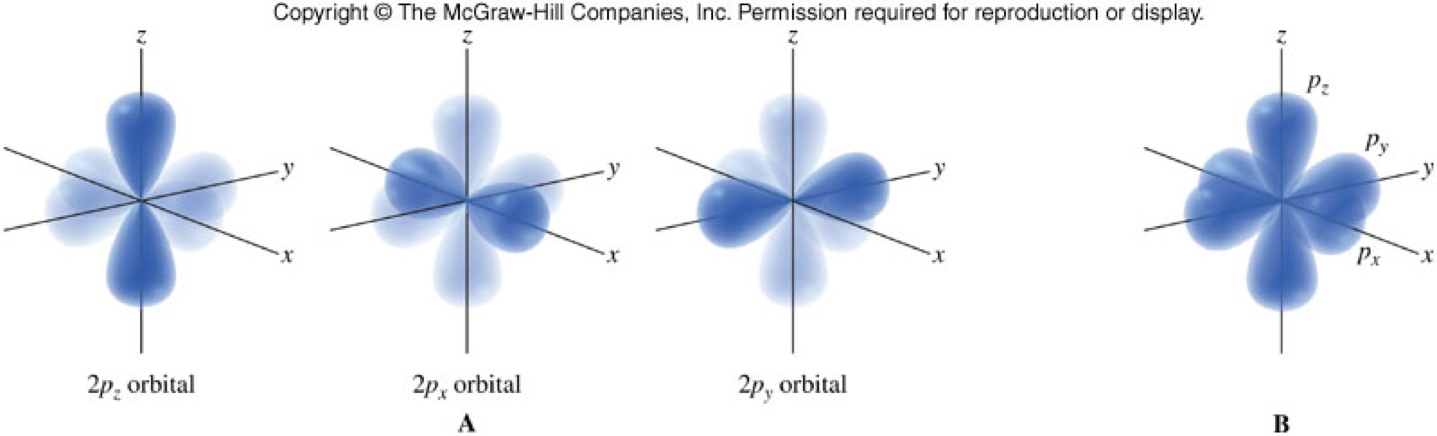
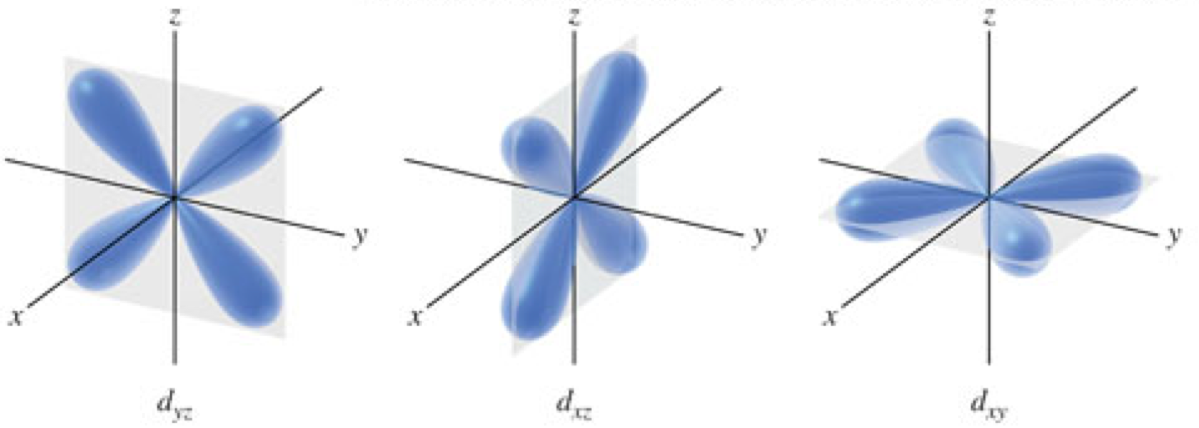
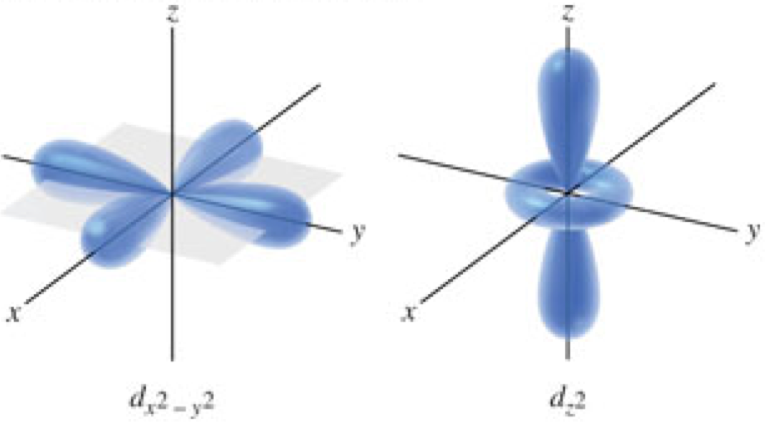
\(d\)-Orbitals
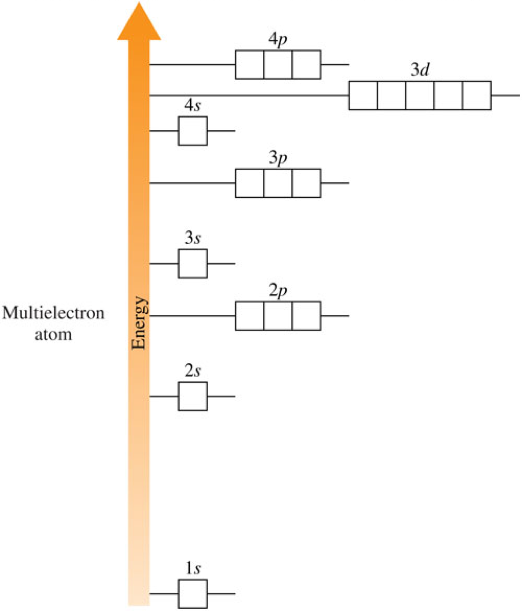
Orbital Diagrams
- Orbital diagrams
- Show the sublevels and orbitals that can exist at each principal energy level
- Each box represents an orbital
- Groups of boxes represent sublevels
Orbital Diagrams Rules
- Two principles and 1 rule determine how the electrons are filled in the principal energy levels and sublevels.
- Aufbau principle
- Electrons fill orbitals starting with the lowest-energy orbitals.
- Pauli exclusion principle
- A maximum of two electrons can occupy each orbital, and they must have opposite spins.
- Hund's rule
- Electrons are distributed into orbitals of identical energy (same sublevel) in such a way as to give the maximum number of unpaired electrons.
- Aufbau principle
Filling Orbitals Diagrams
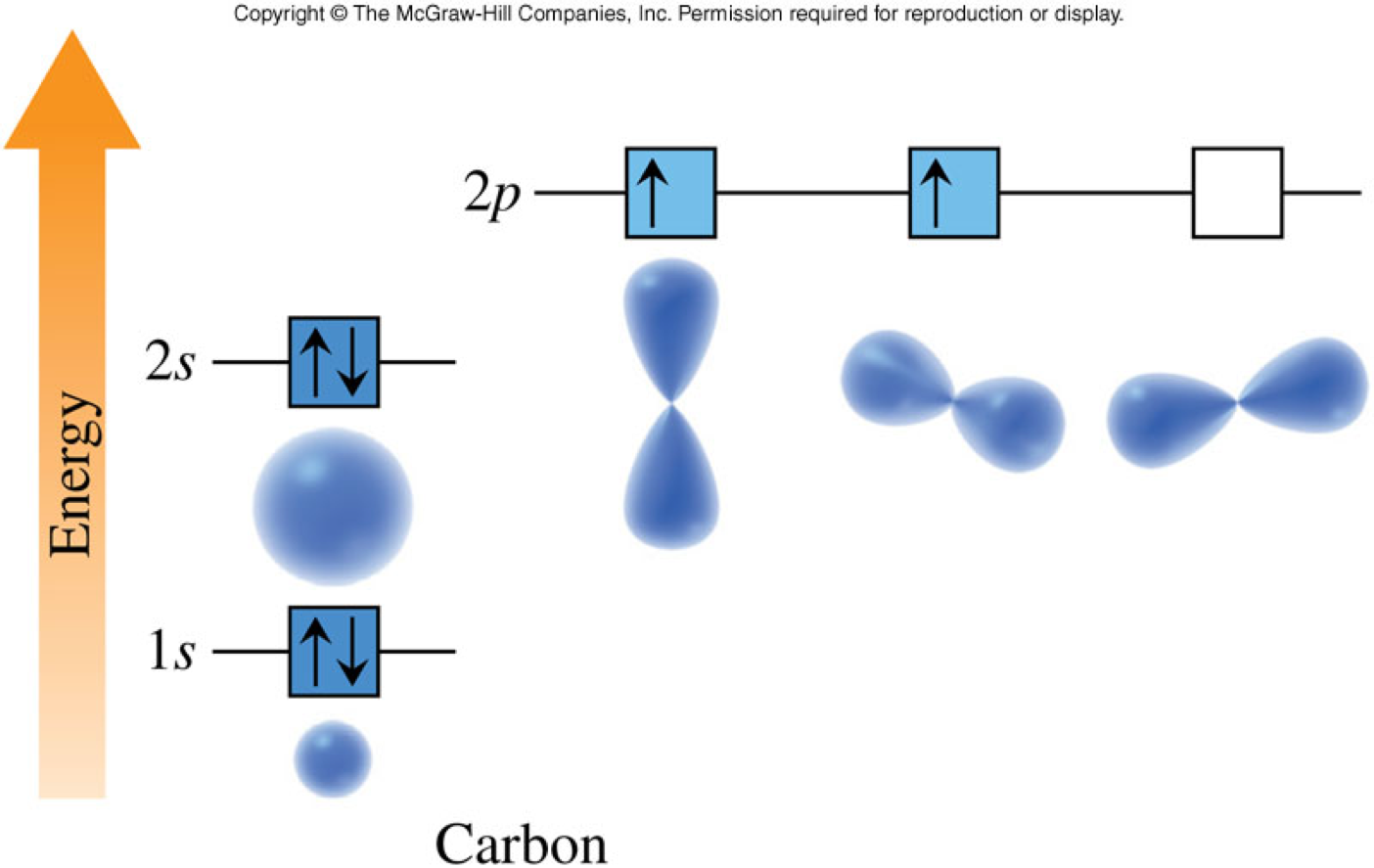
Orbital Diagrams for the 1st Ten Elements
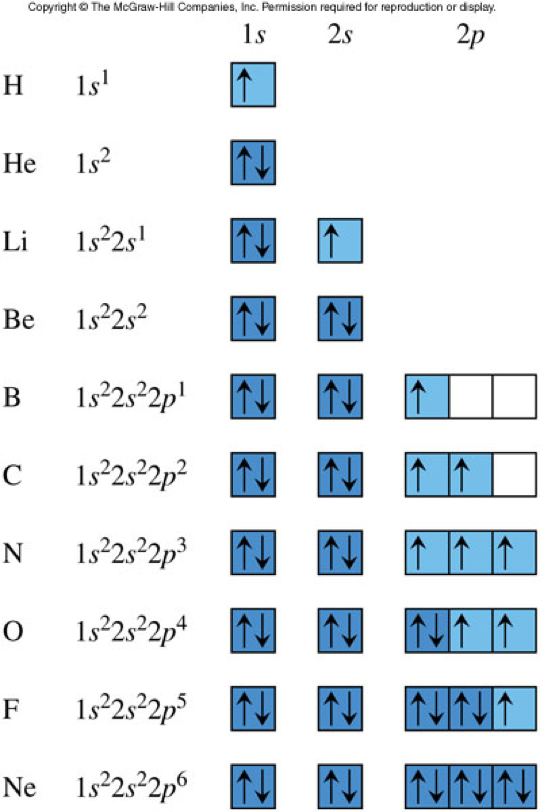
Orbital Diagrams Rules
- Shorthand notation which shows the distribution of electrons among sublevels
- When we write electron configurations, we write the number of the principal quantum number followed by a symbol for the sublevel, and then add a superscript to each sublevel symbol to designate the number of electrons in that sublevel.
- Carbon has 6 electrons. Therefore, using the orbital diagram we obtain: \[ \chem{1s^22s^22p^2} \]

Periodicity of Electron Configurations
- Can you tell the patterns among the following groups of elements?
- Alkali Metals (Group IA (1))
Li \(\chem{1s^22s^1}\) Na \(\chem{1s^22s^22p^63s^1}\) - Alkali Earth Metals (Group IIA (2))
Mg \(\chem{1s^22s^22p^63s^2}\) Ca \(\chem{1s^22s^22p^63s^23p^64s^2}\) - Halogens (Group VIIA (17))
Cl \(\chem{1s^22s^22p^63s^23p^5}\) Br \(\chem{1s^22s^22p^63s^23p^64s^23d^{10}4p^5}\) - Noble Gases (Group VIIIA (18))
Ne \(\chem{1s^22s^22p^6}\) Ar \(\chem{1s^22s^22p^63s^23p^6}\)
- Alkali Metals (Group IA (1))
The Principal Quantum Number and Sublevel on the Periodic Table
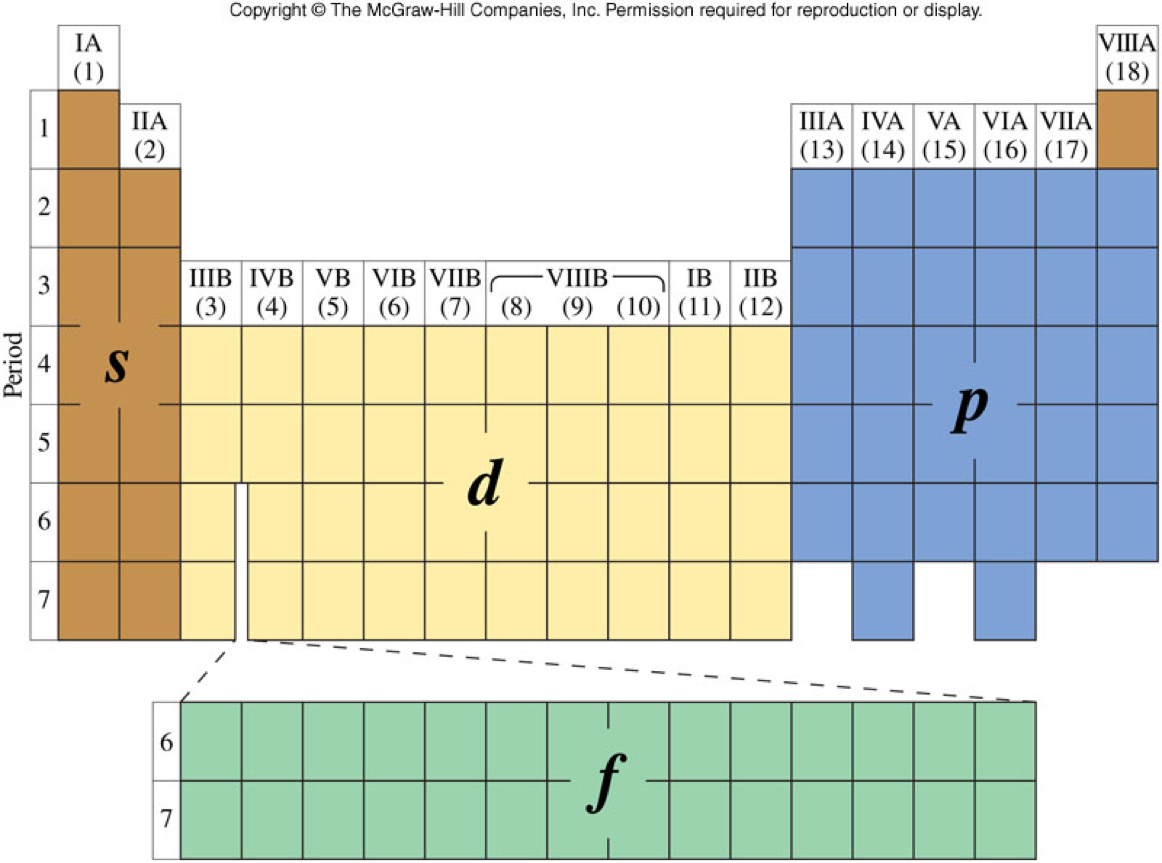
Valence Electrons for Main-Group Elements
- Valence level (shell)
- Last-filled principal energy level
- Highest energy level
- Contains orbitals that are larger than orbitals in lower energy levels
- Valence electron
- An electron that occupies the valence level
- Elements in the same group have the same number of valence electrons
- Example: Br – \( \chem{1s^22s^22p^63s^23p^64s^23d^{10}4p^5}\)
More on Valence Electrons
- Valence electrons
- The Roman numeral group number (which is paired with an A or B).
- The number of valence electrons is also equal to the number of s and p electrons in the valence level for any main-group element.
- Core electron
- An electron in a principal energy level below the valence level
- Inner electron
Abbreviated Electron Configuration
- Starts the electron configuration at the last noble gas before the element in question.
- Write down the noble gas in brackets, then fill in the rest of the electron configuration until you reach the element in question.
- Example:
- Phosphorus (P) has 15 electrons. In the long notation, the electron configuration for P would be: \[ \chem{1s^22s^22p^63s^23p^3} \]
- The abbreviated electron configuration for P would be: \[ \chem{[Ne]3s^23p^3} \]
Electron Configurations for Ions
- Ions form because atoms gain or lose electrons
- Cations
- Positively charged ions
- Subtract the number of the charge from the total number of electrons
- Move to the left the number of spaces equal to the charge on the periodic table
- Anions
- Negatively charged ion
- Add the number of the charge to the total number of electrons
- Move to the right the number of spaces equal to the charge on the periodic table
- Cations
Periodic Trends of Atoms
- Valence electrons are the electrons that participate in chemical reactions because they are the farthest electrons from the nucleus.
- Because elements in the same group have the same number of valence electrons, elements in the same group have very similar reactivities.
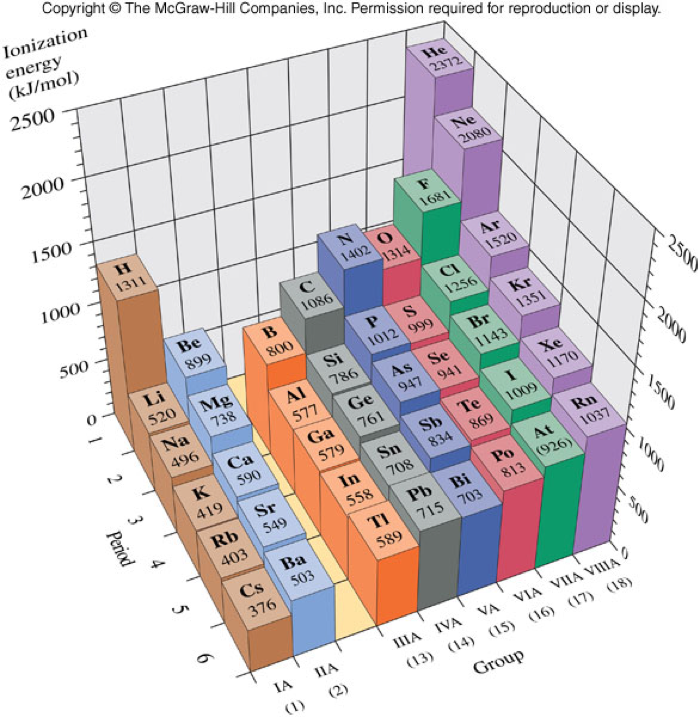
Ionization Energy
- Ionization Energy
- A measure of the energy required to remove a valence electron from a gaseous atom to form a gaseous ion.
- In general, atoms with low ionization energies do not bind their electrons very tightly, and are therefore, very reactive.
- The general trend for ionization energy is for ionization energy to increase from bottom to top and from left to right across the periodic table.
Atomic Size
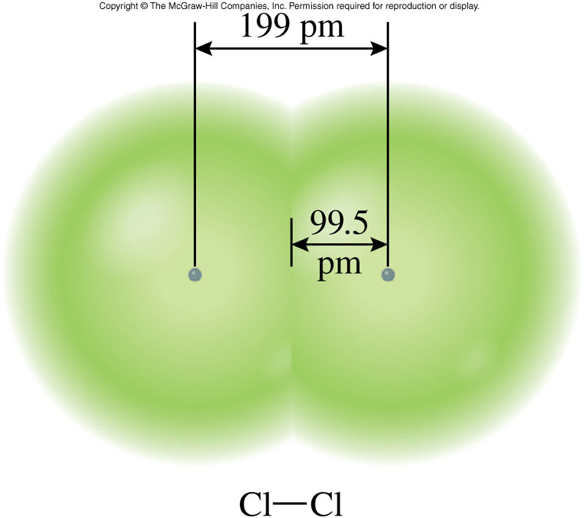
- Atomic size is often described in terms of atomic radius.
- Atomic radius is the distance from the center of the nucleus to the outer edge of the atom.
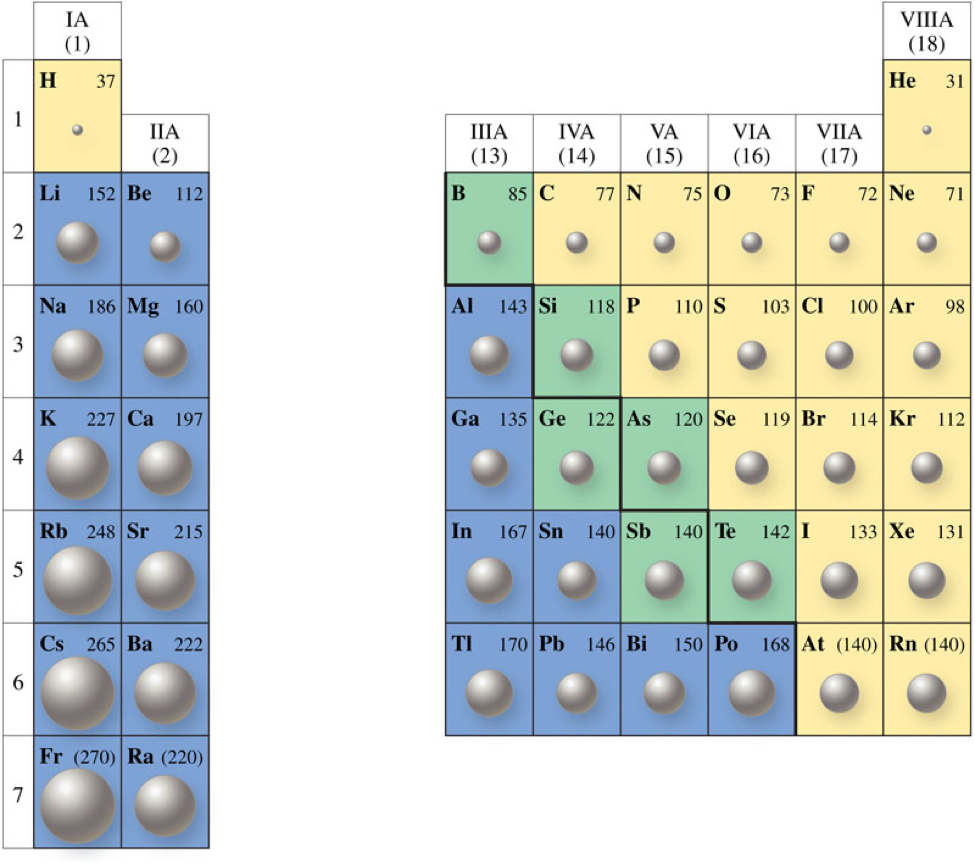
Trends in Atomic Size
- The general trend for atomic size (or radius) is for atomic size to increase from top to bottom and from right to left across the periodic table.
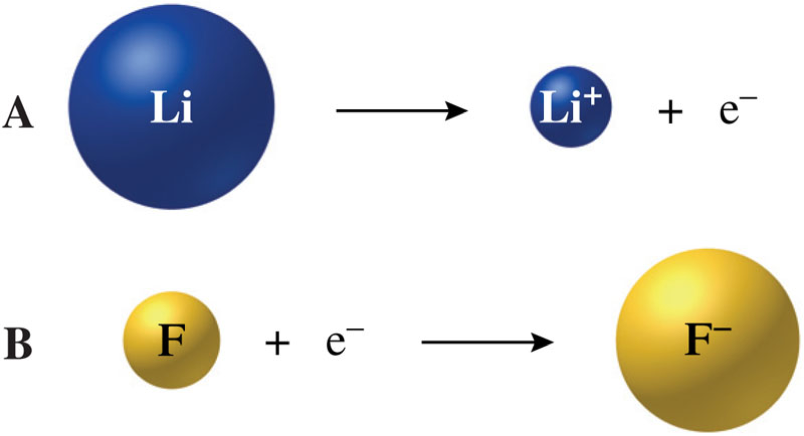
Ionic Size
- Ionic size
- Radius of an ion
- Atoms change radius when they become ions
- The general trend for ionic size (or radius) is for ionic size to increase from top to bottom.
- For cations, as the charge increases, the ionic size decreases.
- For anions, as the charge increases in negativity, the ionic size increases.
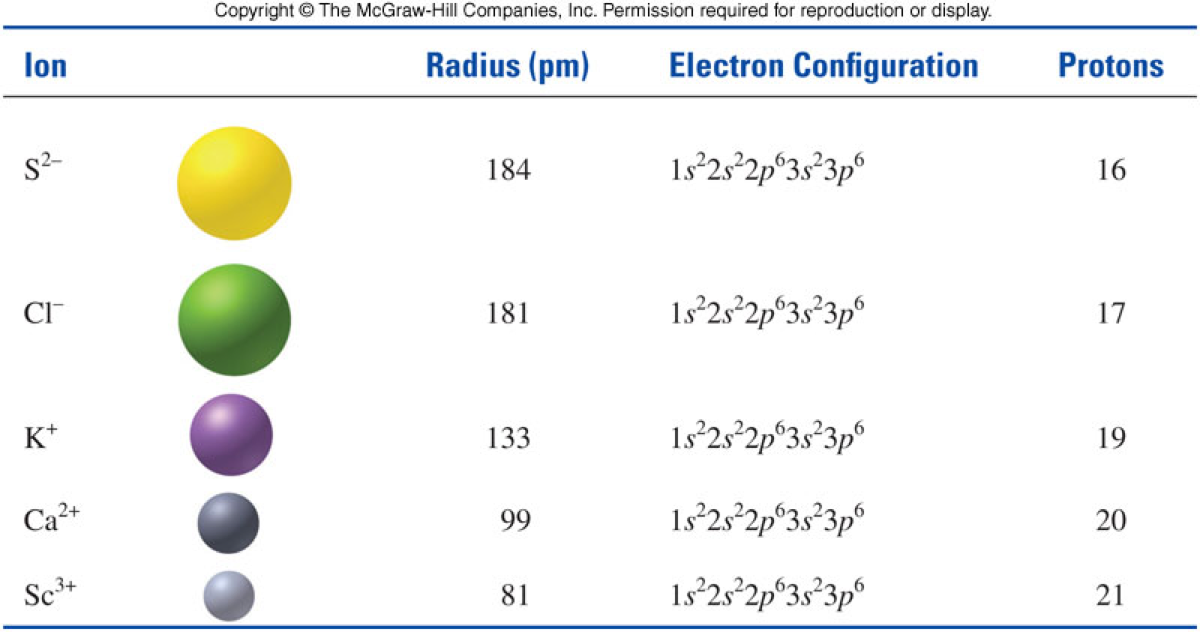
Ionic Size
- For any isoelectronic series, as the number of protons increases, the ion size decreases.
/