Lecture 4
Chemical Composition
Shaun Williams, PhD
The Mole and Molar Mass
The Mole
- The unit that acts as a bridge between the microscopic world and the macroscopic world
- Contains \( 6.022 \times 10^{23} \) particles (molecules, atoms, ions, formula units, etc.)
- This number is called Avogadro's number.
- The amount of substance that contains as many basic particles (atoms, molecules, or formula units) as there are atoms in exactly 12 g of carbon-12
Molar Mass
- Describes the mass of 1 mole of a substance
- We obtain the Molar Mass (MM) from the periodic table by assigning different units to the atomic mass.
- Instead of assigning the atomic mass units of amu, we assign the atomic mass units of grams per 1 mole.
- Molar mass is the conversion factor between mass and moles.
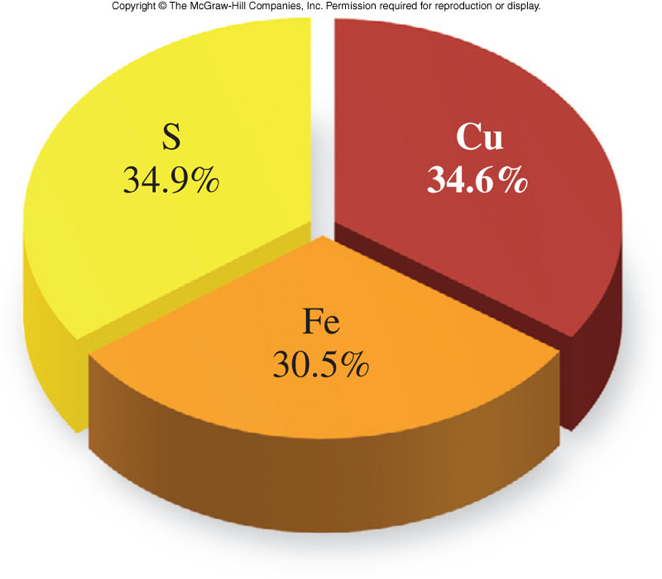
Percent Composition
Percent Composition by Mass
- An expression of the portion of the total mass contributed by each element
- To find the percent composition of E (E is any element):
Conversion with Molar Mass and Avogadro's Number
- To convert from moles to grams or from grams to moles, use a molar mass (MM) as your conversion factor.
- To convert from moles to particles (molecules, atoms, ions, or formula units) or from particles to moles, use Avogadro's number as your conversion factor.
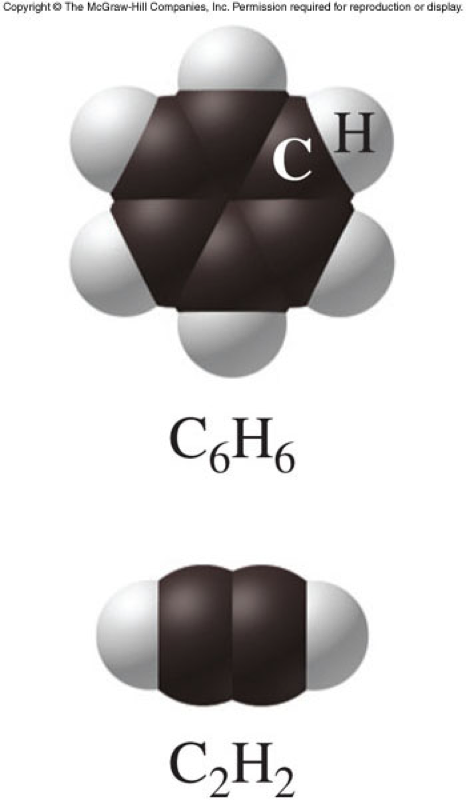
Determining Empirical and Molecular Formulas
Empirical and Molecular Formulas
- Empirical formula
- Expresses the simplest ratios of atoms in a compound
- Written with the smallest whole-number subscripts
- Molecular formula
- Expresses the actual number of atoms in a compound
- Can have the same subscripts as the empirical formula or some multiple of them
Example of Empirical and Molecular Formulas
Empirical and Molecular Formulas - Practice
For which of these substances is the empirical formula the same as the molecular formula?
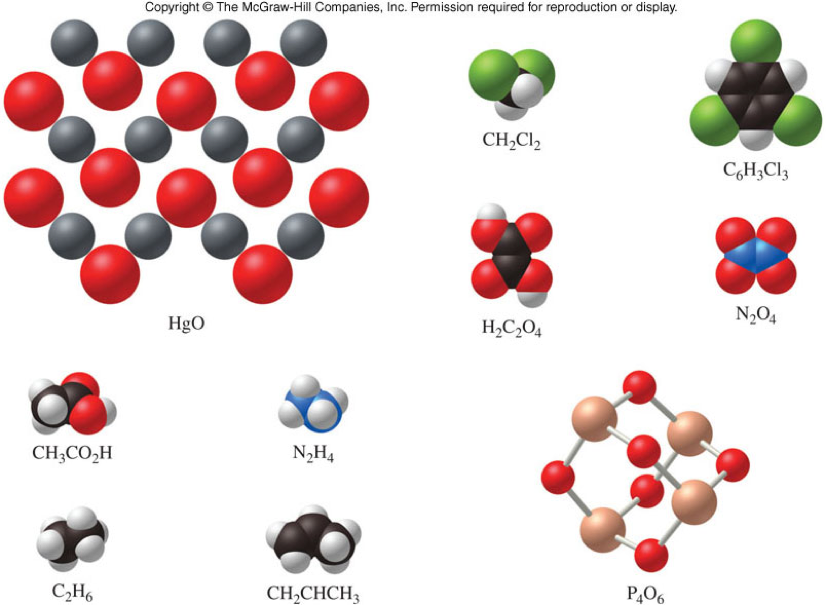
Some Empirical and Molecular Formulas
| Substance | Molecular Formulas | Empirical Formulas |
|---|---|---|
| cyclopentane | \( \chem{C_5H_{10}} \) | \( \chem{CH_2} \) |
| cyclohexane | \( \chem{C_6H_{12}} \) | \( \chem{CH_2} \) |
| ethylene | \( \chem{C_2H_4} \) | \( \chem{CH_2} \) |
| hydrogen sulfide | \( \chem{H_2S} \) | \( \chem{H_2S} \) |
| calcium chloride | This compound does not have a molecular formula | \( \chem{CaCl_2} \) |
Chemical Composition of Solutions
Solutions
- are any homogeneous mixture at the molecular or ionic scale
- are composed of solutes and solvents
- Solutes
- Are present in a lesser amount
- The substances that are dissolved (can be either wet or dry)
- Solvents
- Are present in the larger amount
- The substances that dissolve
- Solutes
Making a Solution
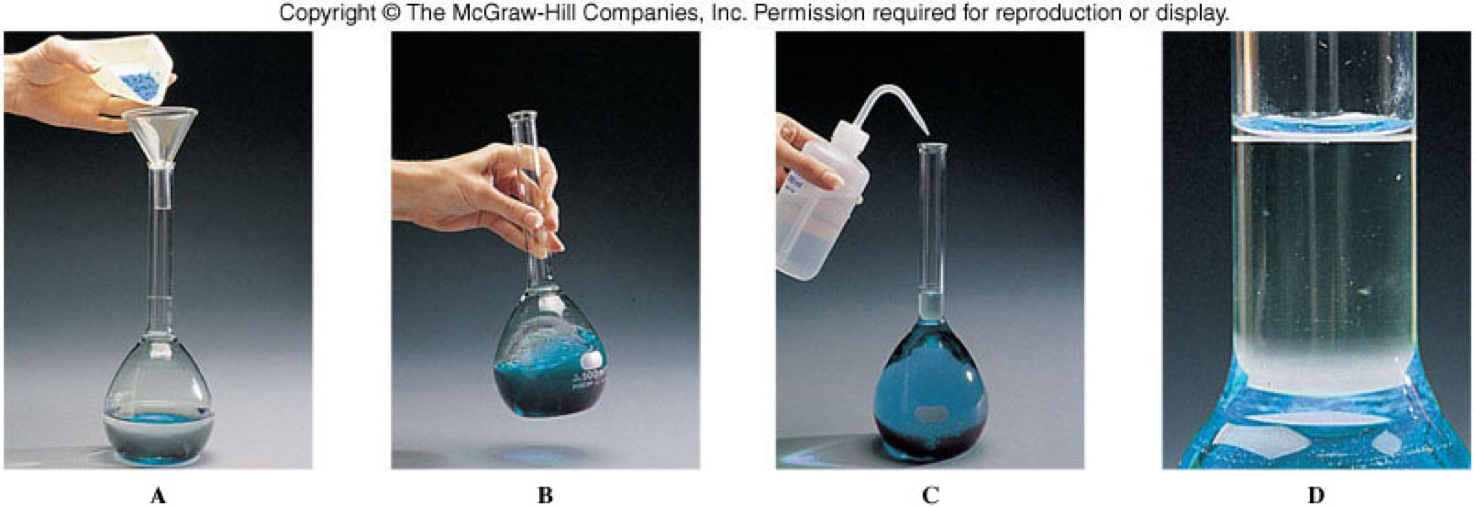
Solution Concentration
- Is the relative amounts of solute and solvent in a solution
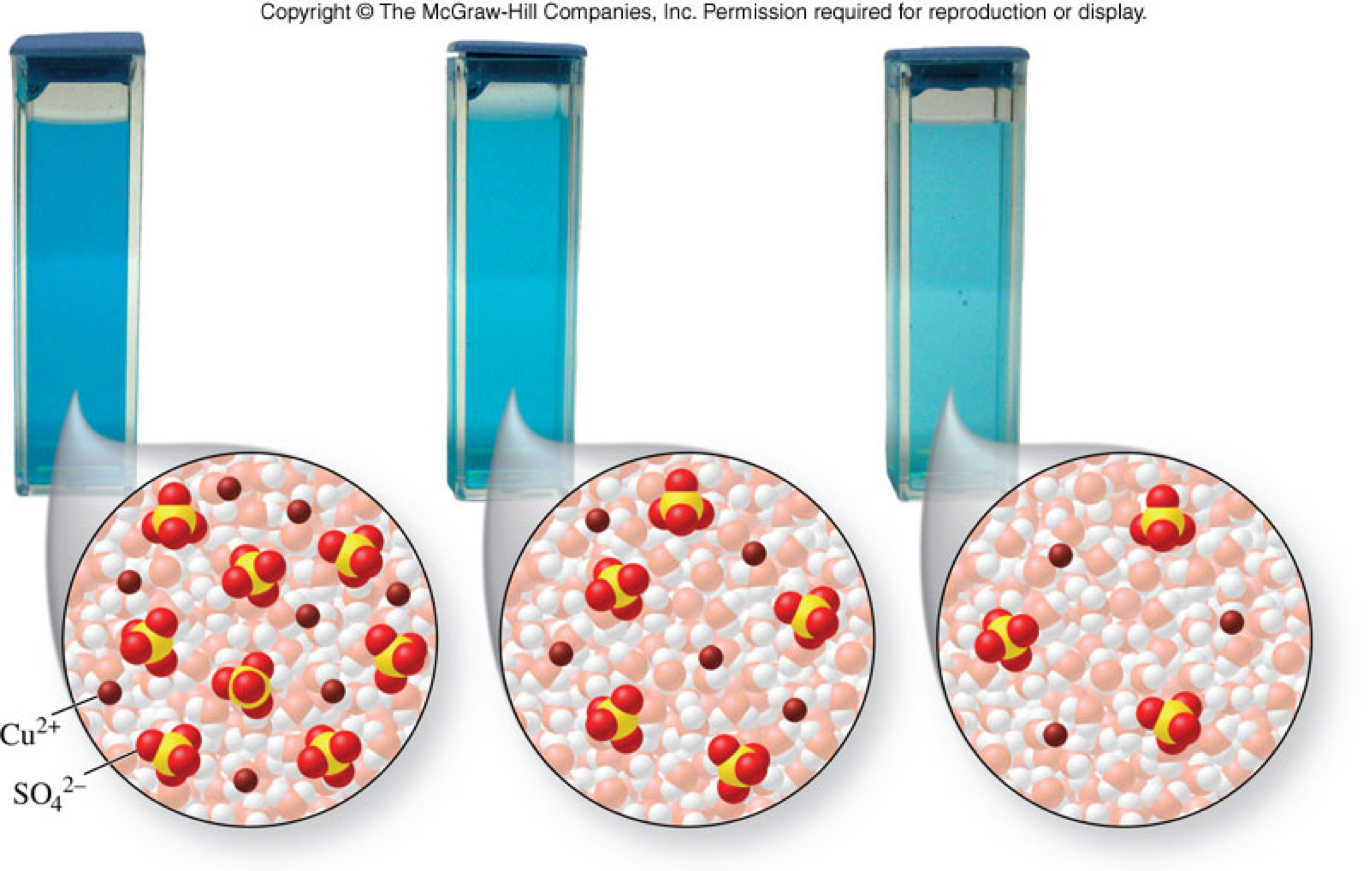
- When compared with one another, solutions are classified as dilute or concentrated.
- Dilute solution
- A solution that contains a relatively small amount of solute
- Concentrated solution
- A solution that contains a relatively large amount of solute
- Dilute solution
Effect of Solution Concentration on Color
Determining Concentration
- Percent by Mass
- Expresses concentration via percentage \[ \%\, mass = \frac{\text{mass solute}}{\text{mass solution}} \times 100\% \]
- Molarity (M)
- The moles of solute dissolved in 1 L of solution
- The most common units of concentration \[ M = \frac{\text{moles solute}}{\text{Liters of solution}} \]
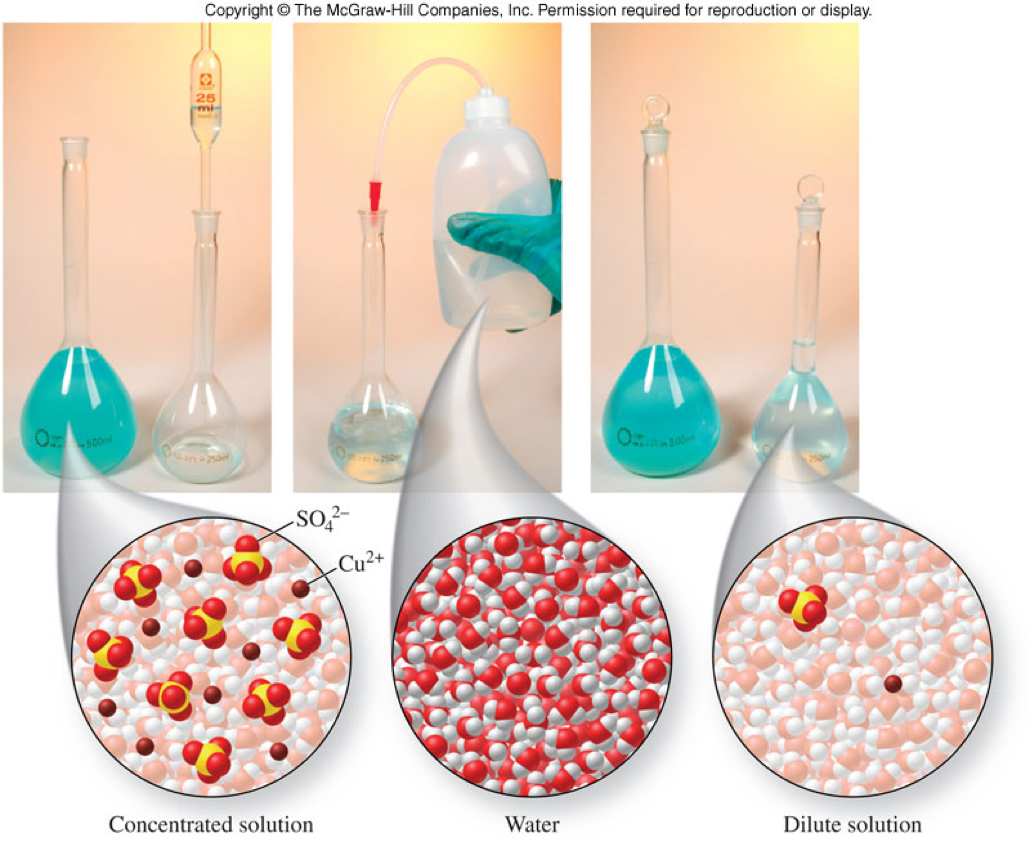
Dilution and the Dilution Equation
- Dilution
- The process of adding more solvent to solution
- The dilution equation: \[ M_{con} V_{con} = M_{dil}V_{dil} \] \[ M_1V_1 = M_2V_2 \]

Molecular View of Dilution
/