Lecture 10
The Liquid and Solid States
Shaun Williams, PhD
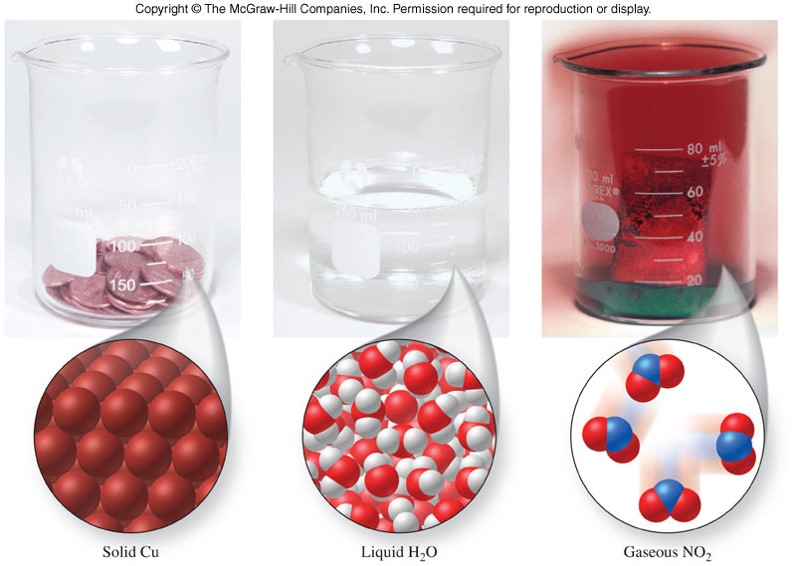
Examples of Solids, Liquids, and Gases
Changes of State
- Transitions between these states
- Also called phase changes
- 6 main phase changes:
- Evaporation (also called vaporization)
- Condensation
- Freezing
- Melting (also called fusion)
- Sublimation
- Deposition
Liquid-Gas Phase Changes
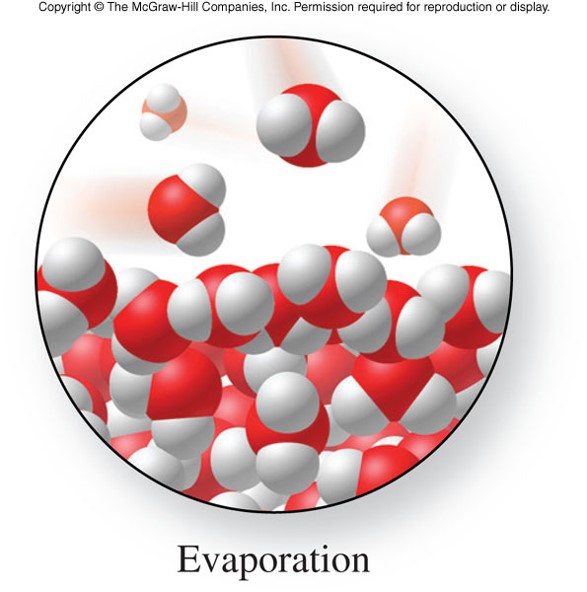
- Evaporation (vaporization)
- At the surface of a liquid, some molecules may have sufficient kinetic energy to escape into the gas state.
- Heat is required to maintain the temperature needed for evaporation.
- Evaporation is an endothermic process.
Liquid-Gas Phase Changes (cont.)
- Condensation
- Transition from a gas to a liquid
- Occurs when gas particles cannot escape the container and thus, come into contact with a liquid
- An exothermic process (energy is released)
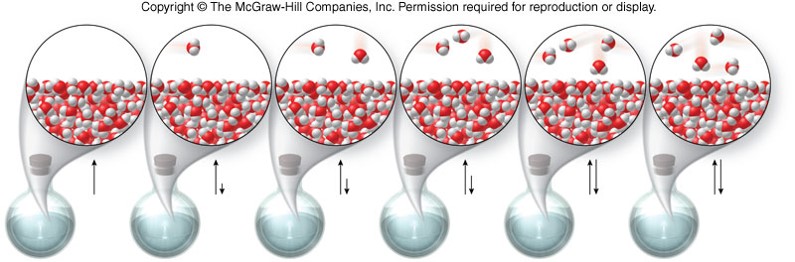
Equilibrium
- A state in which opposing processes occur at equal rates
- An equilibrium is designated by a double arrow, such as: \[ \text{liquid} \rightleftharpoons \text{gas} \]
- In the above equilibrium, the rates of evaporation and condensation are equal.
- The gas produced by evaporation exerts a pressure on the liquid below it.
- At equilibrium, this pressure is called vapor pressure.
- Vapor pressure increases as temperature increases.
- At equilibrium, this pressure is called vapor pressure.
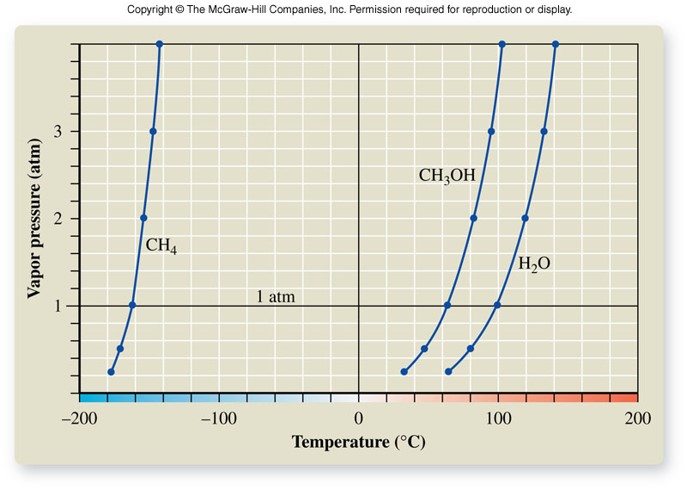
Boiling Point
- The temperature at which boiling occurs
- Boiling occurs when the vapor pressure equals the external pressure of the atmosphere
- Normal boiling point
- Occurs when the atmospheric pressure is exactly 1 atm
Liquid-Solid Phase Changes
- Freezing
- The average kinetic energy of a liquid molecules fall when it is cooled
- If the \( KE_{av} \) falls low enough, then the molecules become fixed in position into the solid
- Therefore, freezing is the conversion of a liquid into a solid
Liquid-Solid Phase Changes (cont.)
- Freezing point
- The temperature at which the freezing of a liquid into a solid state occurs
- As with boiling temperature, the two states are in equilibrium with one another. \[ \text{solid} \rightleftharpoons \text{liquid} \]
- Normal freezing point
- The temperature when the freezing equilibrium is achieved under a pressure of 1 atm.
- The temperature at which the freezing of a liquid into a solid state occurs
Freezing
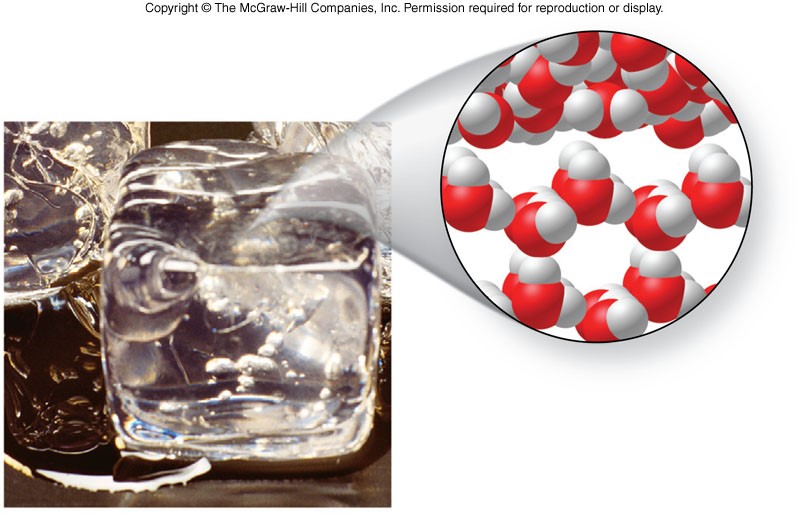
Melting
- Melting
- Phase change from solid to liquid
- Reverse of freezing
- Opposite process of freezing
- Also called fusion
- Melting point
- Same temperature as freezing point
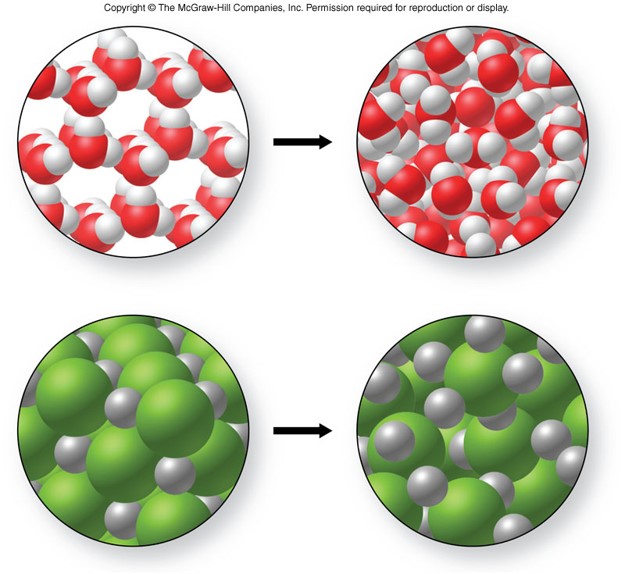
Solid-Gas Phae Changes
- Sublimation
- Evaporation of a solid
- Occurs when a solid has a high vapor pressure
- Solid can change directly from a solid to a gaseous state without going through the liquid state
- Deposition
- Can go directly from gas to solid without passing through the liquid state
- Reverse of sublimation
Cooling Curve
- Shows the phase changes of a substance in a graph plotting temperature versus heat removed at constant pressure
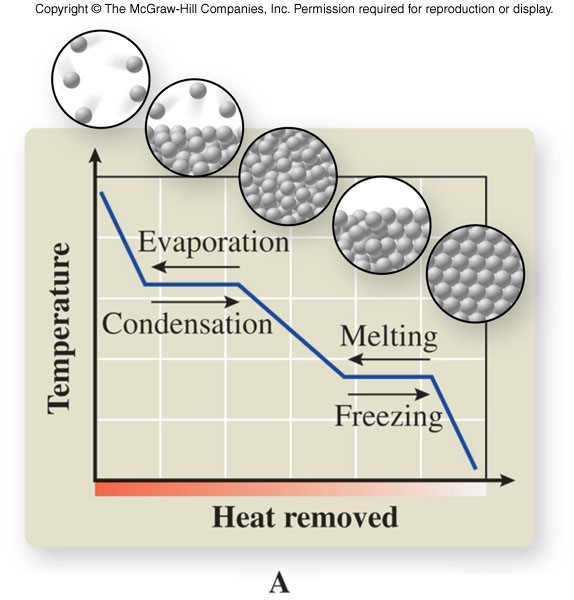
Heating Curve
- Shows the phase changes of a substance in a graph plotting temperature versus heat added at constant pressure
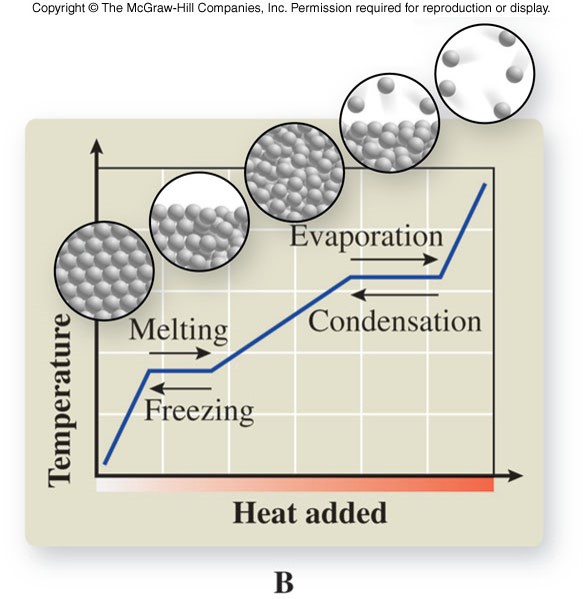
Energy Changes
- Each change of state takes place at constant temperature
- Any phase change is accompanied by an energy change
- The change in energy for each process is called the heat of that process (\(q\)) \[ q=m \times C \times \Delta T \]
- Molar heat of fusion
- Energy required to melt 1 mole of a substance
- Molar heat of vaporization
- Energy required to evaporate 1 mole of a substance
Intermolecular Forces
- An attractive force that operates between molecules
- There are many kinds of intermolecular forces:
- London dispersion force
- Dipole-dipole force
- Hydrogen-bonding force

The Forces
| Type of Force | Type of Interaction | Occurrence |
|---|---|---|
| London dispersion force | A temporary dipole in one molecule induces the formation of a temporary dipole in a nearby molecule and is attracted to it. | All atoms and molecules |
| Dipole-Dipole Force | Polar molecules (permanent dipoles) attract one another | Polar molecules |
| Hydrogen-Bonding Force | Two dipoles, one containing hydrogen to an electronegative element and the other containing an electronegative element, attract one another. | Polar molecules containing unpaired molecules and a hydrogen bonded to nitrogen, oxygen, or fluorine |
London Dispersion Forces
- Instantaneous dipole
- A temporary dipole formed when the electrons in an atom or nonpolar molecule happen to be more on one side in an instant in time, causing it to be more negative than normal and the opposite side positive
- Induced dipole
- Positive end of the dipole exerts an attractive force on nearby electrons, causing an adjacent atom to develop into another temporary dipole
London Dispersion Forces (cont.)
- London dispersion force
- The attraction between temporary dipoles
- Occurs between atoms and molecules
- Only intermolecular force in nonpolar substances
- Tend to be stronger the larger the atom or molecule
- Relatively weak forces
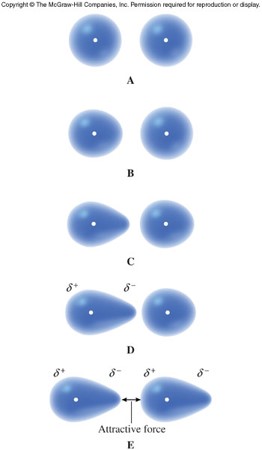
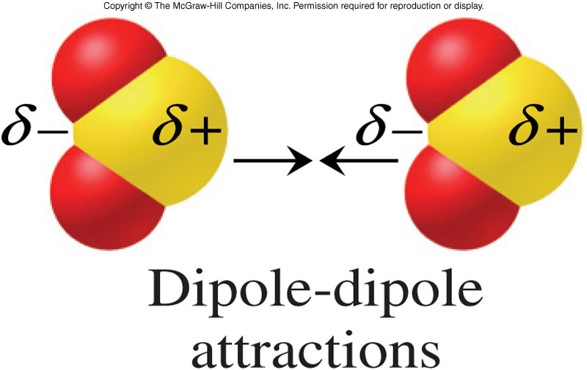
Dipole-Dipole Forces
- Attraction between polar molecules
- Occurs when the partially positive end of one molecule attracts the partially negative end of another molecule
- Generally stronger than London dispersion forces
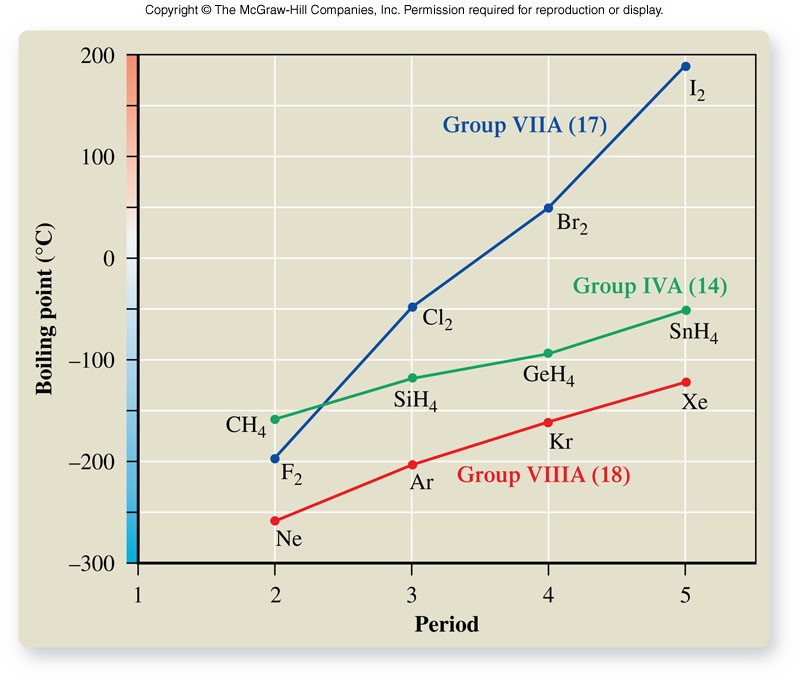
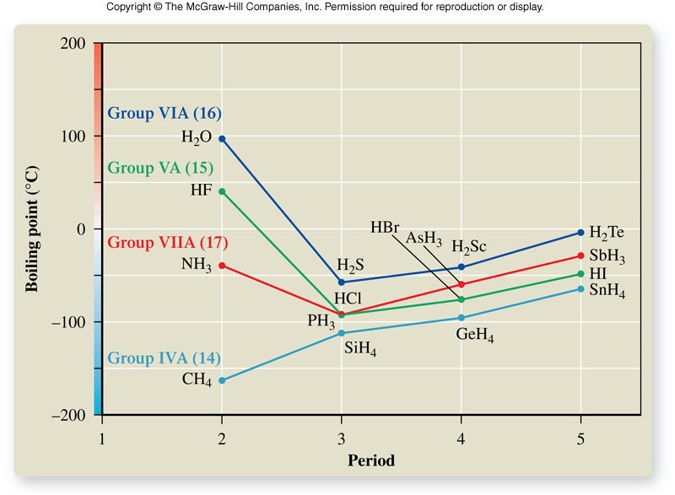
Boiling Points
Hydrogen Bonding
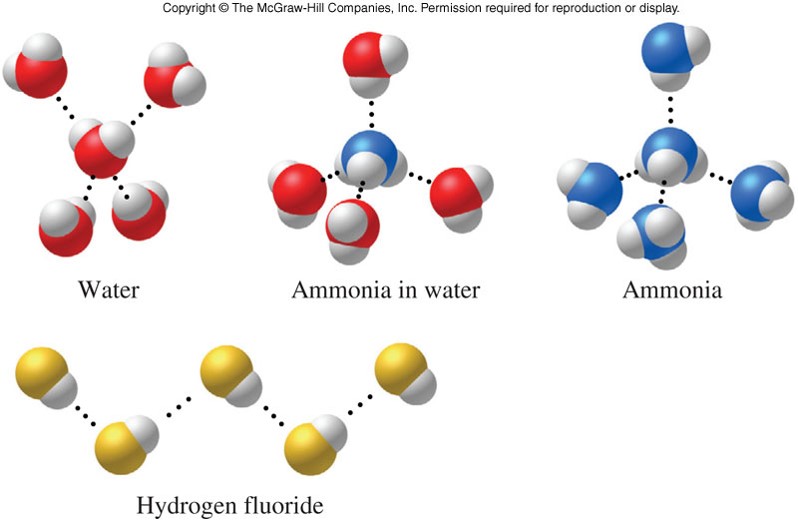
- Special type of dipole-dipole force
- Only occurs in molecules that contain hydrogen bonded to a small, highly electronegative element
- Stronger than a regular dipole-dipole force
- Important force in living systems by stabilizing molecular shapes
Hydrogen Bonding Examples
Trends in Intermolecular Forces
- Remember:
- London forces exist between all atoms and molecules.
- Dipole-dipole moments exist in only polar compounds.
- Hydrogen bonds only exist in polar compounds that contain hydrogen.
- In terms of strength (magnitude), intermolecular forces compare as shown below:
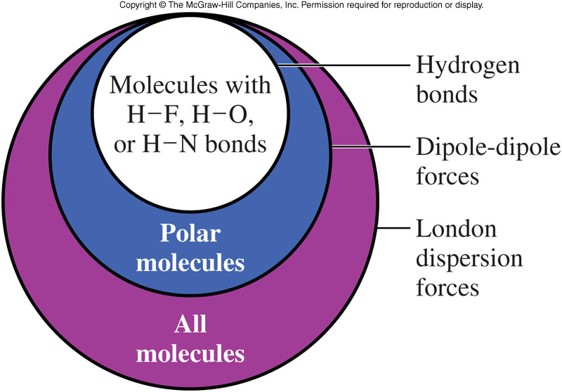
\[ \text{London Disp.} \lt \text{Dipole-Dipole} \lt \text{Hydrogen Bonds} \]
Properties of Liquids
- Are related to the distance between particles and to intermolecular forces
- Particles in a liquid are much closer together than the particles in a gas
- Liquid particles are not fixed, as they are in a solid
- Three common properties of liquids:
- Density
- Viscosity
- Surface tension
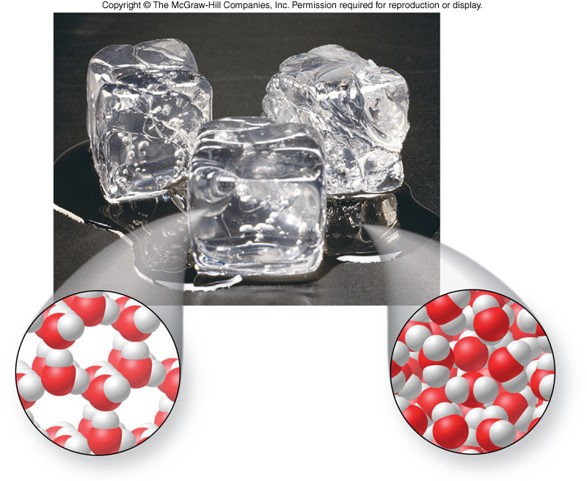
Density
- Remember: \[ \text{density}=\frac{\text{mass}}{\text{volume}} \]
- Densities of the states of matter are related to the distance between particles
- Most substances are denser as solids than as liquids because their molecules or atoms are closer together
- Water is an exception in that ice is less dense than liquid water
Viscosity
- Liquids and gases are fluids.
- A fluid is any substance that can flow
- Viscosity is the resistance of a substance to flow.
- Generally, the viscosity of liquids is low
- Viscosities generally vary by increasing with the magnitude of their intermolecular forces and with molecular size.
Surface Tension
- The amount of work required to increase the surface area of a liquid by a unit amount
- Causes a liquid surface to behave like a stretched membrane
- The greater the intermolecular forces in a liquid, the greater the surface tension
- Surface tension decreases as temperature increases
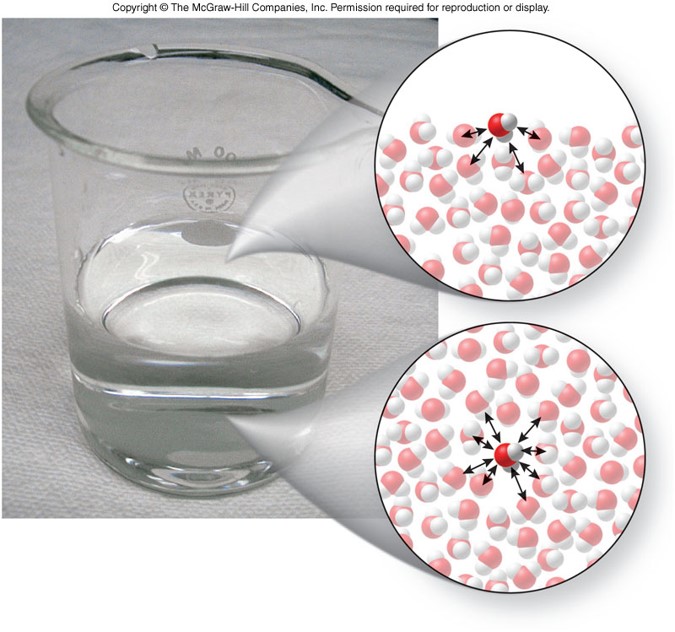
Meniscus
- Either a concave or convex curved surface of a liquid produced by intermolecular forces
- A concave surface occurs when the intermolecular forces between the liquid and the glass are greater than the intermolecular forces among the liquid molecules
- A convex surface occurs when the intermolecular forces between the liquid and the glass are less than the intermolecular forces among the liquid molecules
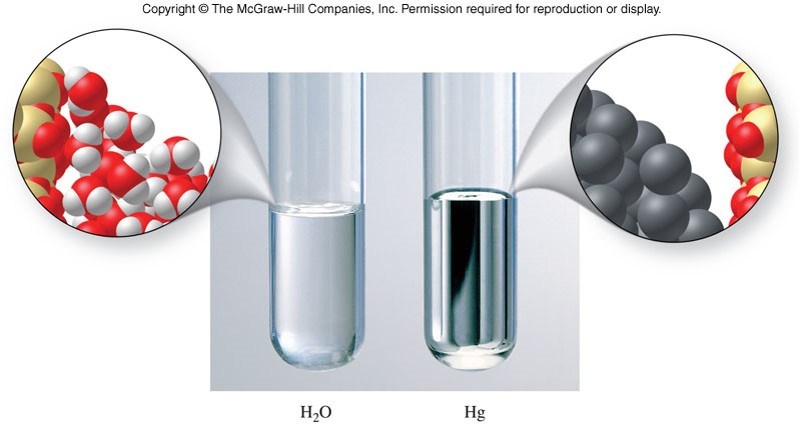
Properties of Solids
Amorphous and Crystalline Solids
- Amorphous solid
- Occurs when the temperature of a liquid drops rapidly, resulting in the particles solidifying in a partially disordered state
- Particles are somewhat randomly arranged
- Lacks regular form
- Crystalline solid
- Occurs when a liquid solidifies slowly, allowing the array of particles to become well ordered
- Most solids are of this type
- Results in the symmetrical arrangement of planar faces
Amorphous and Crystalline Solids Examples

Crystals and Crystal Lattices
- Crystal
- An orderly, repeating, three-dimensional assembly of fundamental particles (atoms, molecules, or ions)
- Crystal lattice
- Pattern forms by repeating arrays of crystal
- Also called crystal structure
- Atoms, molecules, or ions are arranged in close-packed structures
- Orderly arrangement that is an efficient way to use space
Closest-Packed Arrangements

Types of Crystalling Solids
- Solids show a wide range of properties such as:
- Melting point
- Hardness
- Malleability
- The types of forces holding the fundamental particles together help explain the properties
- 4 Types of crystalline solids
- Metallic solids
- Ionic solids
- Molecular solids
- Network solids
Types of Crystals
| Type of Solid | Fundamental Particles | Attractive Forces | Properties |
|---|---|---|---|
| Metallic | Atoms | Attractions between nuclei and delocalized electrons | Low melting point & soft; or high melting point & hard; good heat & electrical conductors; malleable & ductile |
| Ionic | Cations and Anions | Ionic bonds | High melting point; hard, brittle; nonconductors when solid; electrical conductors when melted |
| Molecular | Polar Molecules | Dipole-dipole forces | Low to moderate melting point; variable hardness; may be brittle; nonconductors |
| Nonpolar Molecules | London dispersion forces | Low melting point; soft; poor heat conductors; electrical insulators | |
| Netword | Atoms | Covalent bonds | Very high melting point; very hard; somewhat brittle; non- or semiconductors |
Metallic Solids
- Valence electrons move freely through all parts of a metal
- Attractions between atoms of a metal are delocalized, and therefore it is easy to move atoms by applying force
- Ductile and malleable
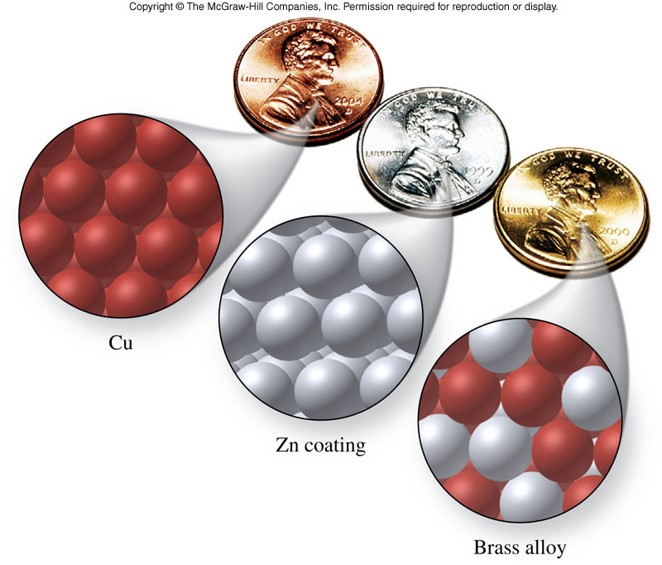
Alloys
- Forms when a metal is mixed with one or more additional metallic or nonmetallic elements
- Have properties different than those of their parent elements
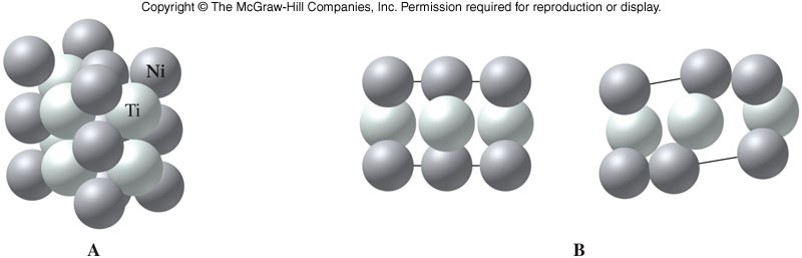
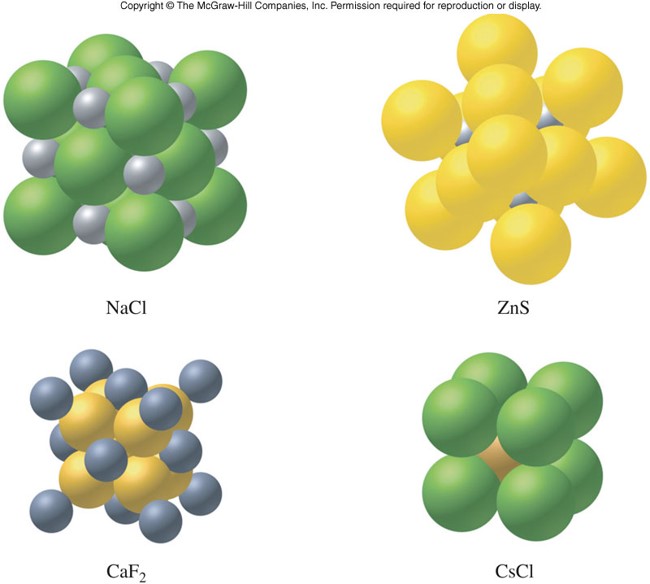
Ionic Solids
- Contain cations and anions arranged in crystalline solids
- Electrostatic forces (ionic bonds) hold together ionic crystals
- High melting points
- Hard and brittle
- Solids are not electrical conductors, but melted or dissolved, they become good conductors
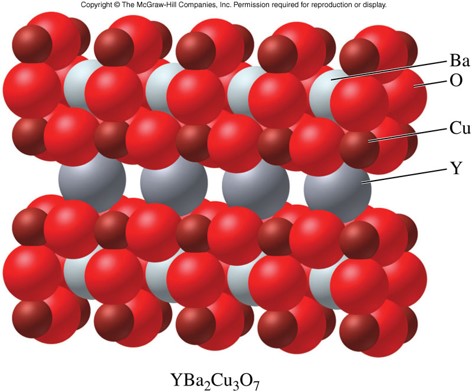
Superconductors
- Offer no resistance to the conduction of electrical current
- Repel magnetic fields
- Although the nature of superconductors is not entirely understood, the crystal structure of the solid material does play a role
- Often formed from silicon and/or other metalloids
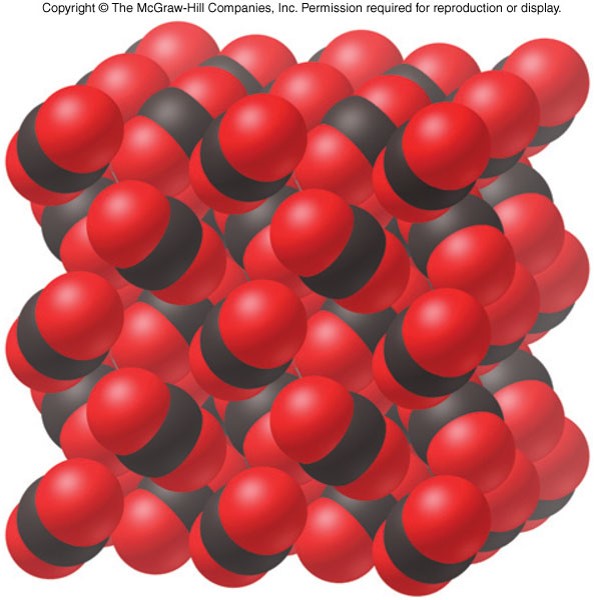
Molecular Solids
- Intermolecular forces between molecules hold a molecular solid together
- Nonpolar solids are held together by London dispersion forces
- Form soft crystals with low melting points
- Electrical insulators
- Poor conductors of heat
- Polar solids are held together by London dispersion forces and dipole-dipole forces
- Typically harder than nonpolar solids
- Low to moderate melting points
- Electrical insulators (no ions)
- Nonpolar solids are held together by London dispersion forces
Example of a Molecular Solid
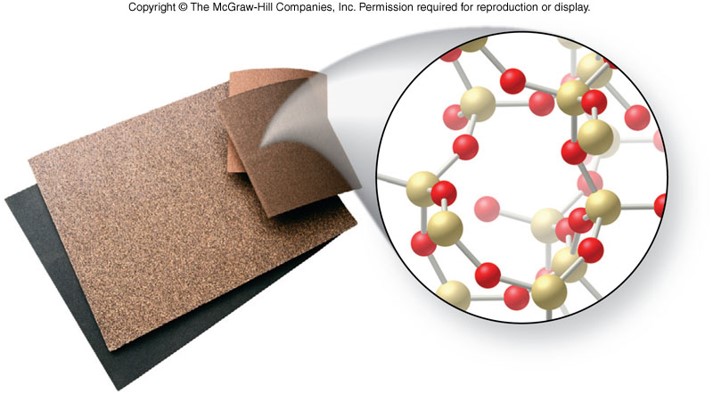
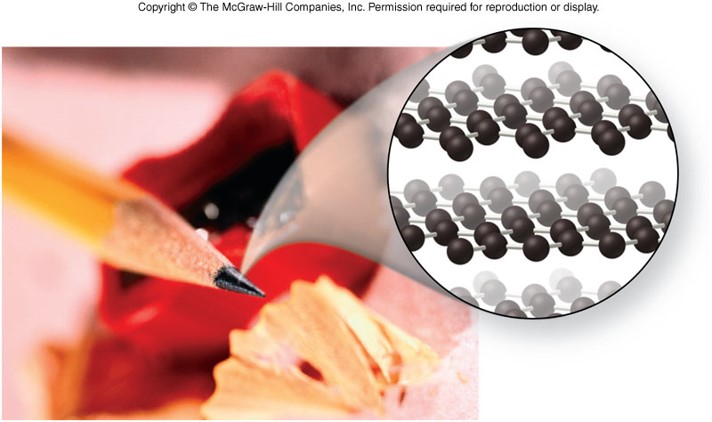
Network Solids
- Consists of a giant molecule that forms the entire crystal
- Formed by metalloids or carbon
- Strong covalent bonds connect the atoms in a network solid
- Poor electrical conductors
- High melting points
- Very hard
- Have very stable, three–dimensional structures
- Many have a diamond structure, or some derivative of it

Example of a Network Solids
/