Chapter 2
Atoms, Ions, and the Periodic Table
Shaun Williams, PhD
Dalton's Atomic Theory
Law of Conservation of Mass
- the mass of the products (overall) always equals the mass of the reacting substances
- Proposed by Antoine Lavoisier in 1787
- His experiments showed that no measurable change in mass occurs during a chemical reaction.
Law of Conservation of Mass - Experiment

Law of Definite Proportions
- Proposed by Joseph Proust between 1797 and 1804
- states that all samples of the same compound always contain the same proportions by mass of the component elements
- For example, water is always composed of oxygen and hydrogen in a mass ratio of 8:1 (or 16:2).
Dalton's Atomic Theory
- Dalton's Atomic Theory has 4 postulates:
- All matter is composed of exceedingly small, indivisible particles called atoms.
- All atoms of a given element are identical both in mass and in chemical properties. However, atoms of different elements have different masses and different chemical properties.
- Atoms are not created or destroyed in chemical reactions.
- Atoms combine in simple, fixed, whole-number ratios to form compounds.
Atomic Force Microscope Image of a Gold Surface
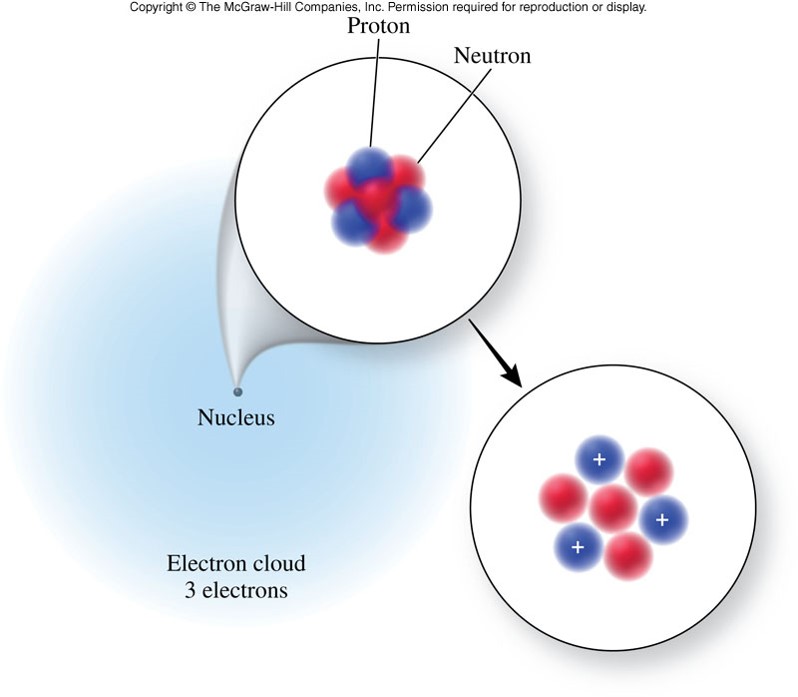
Structure of the Atom
The Inner Structure of the Atom
- Atoms actually are divisible. They are composed of subatomic particles.
- Subatomic particles include:
- 1 kind of particle found outside the nucleus
- Electrons - negatively charged subatomic particles
- 2 kinds of particles found in the nucleus (center of the atom)
- Protons - positively charged subatomic particles
- Neutrons - uncharged subatomic particles
- 1 kind of particle found outside the nucleus
A Graphic of the Atom
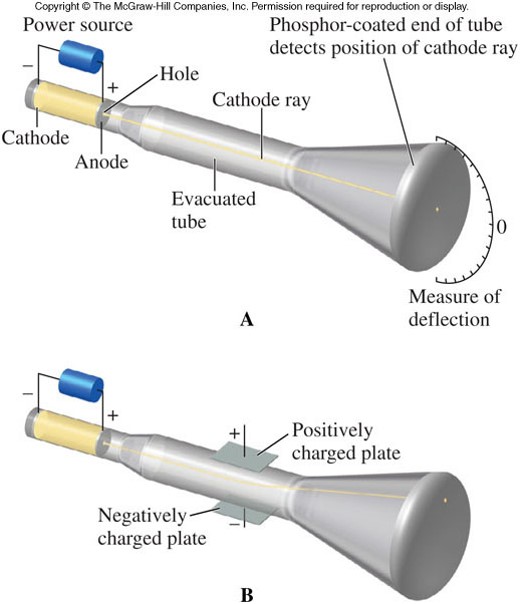
The Discovery of Electrons
- The existence of the electron was demonstrated by J.J. Thomson in 1897.
- He conducted a series of experiments with cathode ray tubes, in which:
- Voltage was applied by connecting each end of a tube to a battery.
- The electricity forms rays that flow from one end of the tube to the other and that are visible through specially coated glass.
- When an electric or magnetic field was applied to the tube (and the rays), the rays bent toward a positively charged plate, and were deflected by a negatively charged plate. Because like charges repel and opposite charges attract, the particles were negatively charged.
- He conducted a series of experiments with cathode ray tubes, in which:
The Cathode Ray Tube
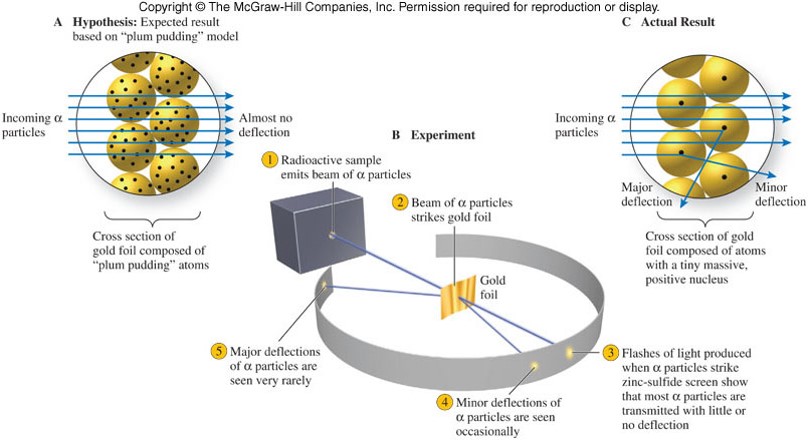
The Nuclear Atom
Subatomic Particles
- Protons have:
- a charge equal to \( +1.6022 \times 10^{-19}\, C \) (expressed as +1)
- a mass equal to \( 1.6726 \times 10^{-24}\, g \) (approx. the same mass as a hydrogen atom)
- Neutrons have:
- no charge
- a mass equal to \( 1.6749 \times 10^{-24}\, g \)
- Neutrons were proposed by Ernest Rutherford in 1907 (to account for a mass discrepancy in the nucleus) and discovered in 1932 by James Chadwick.
Subatomic Particles - Continued
- Electrons have:
- a charge equal to \( -1.6022 \times 10^{-19}\, C \) (expressed as -1)
- a mass equal to \( 9.1094 \times 10^{-28}\, g \) (1836 times less than the mass of one hydrogen atom)
- Electrons were discovered in 1897 by J.J. Thomson.
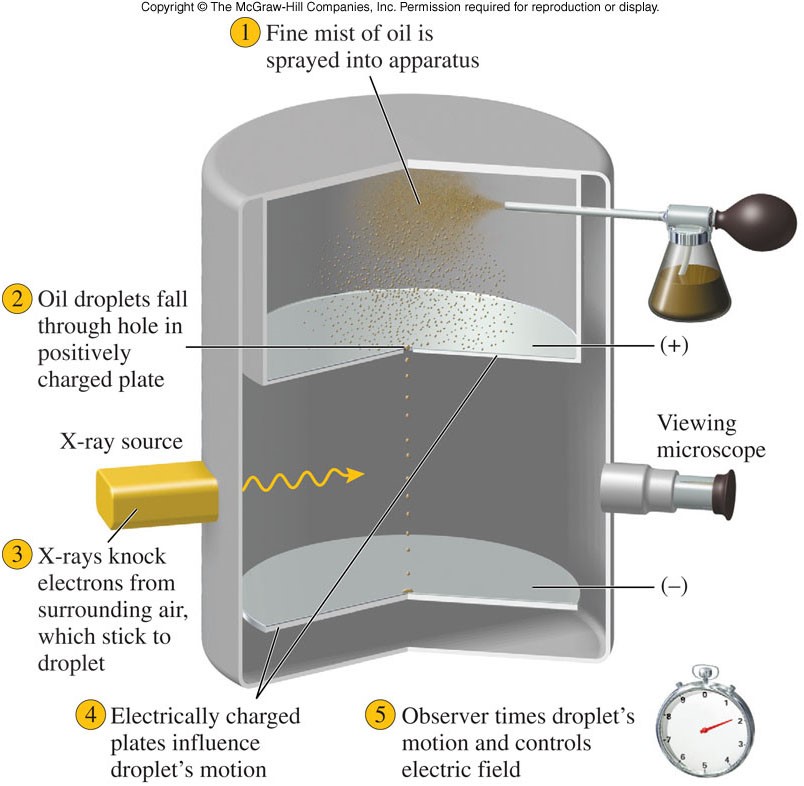
Oil Drop Experiment
Atomic Number and Mass Number
- Atomic Number (Z)
- the number of protons in the nucleus of an element's atom
- is generally found on the periodic table above the elemental symbol
- Mass Number (A)
- the number of protons and neutrons in the nucleus of an element's atom
- is generally found below the elemental symbol on the periodic table \[ \chem{A=Z+N} \]
- Neutron Number (N)
- the number of neutrons in the nucleus of an element's atom
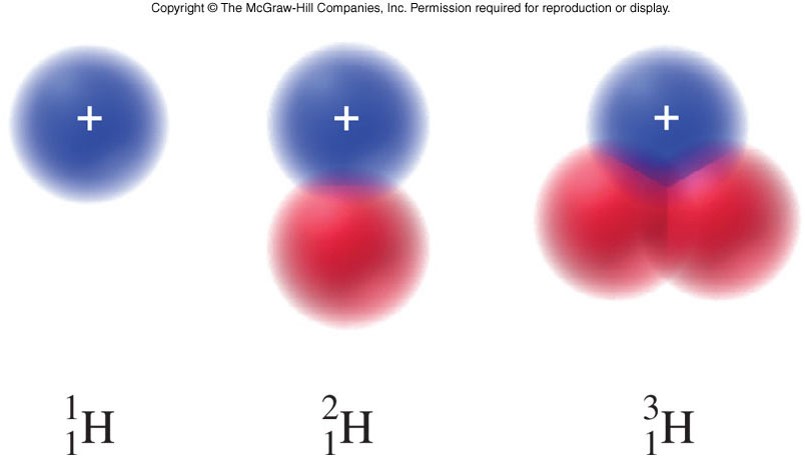
Isotopes
- An isotope of an element
- is an atom that contains a specific number of neutrons.
- Many elements have multiple isotopes.
- Specific isotopes have many applications, particularly in medical testing, imaging, and treatment.
- An isotope symbol
- is a common notation that represents the mass number, atomic number, and elemental symbol.
- The subscript in the isotope symbol is the atomic number.
- The superscript in the isotope symbol is the mass number.
Isotopes, Hydrogen, Deuterium, and Tritium
Ions
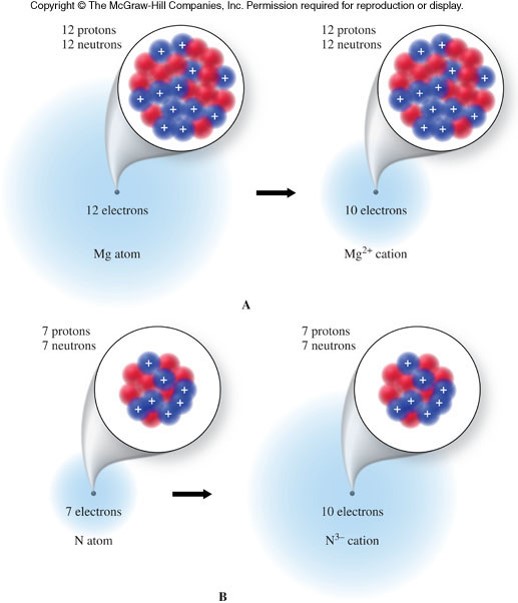
What about different numbers of electrons?
- An ion
- is a charged atom that contains more or less electrons than protons.
- The overall charge is represented as a superscript to the right of the elemental symbol.
- Ions can be classified as cations or anions.
- Cations
- are ions with a positive charge
- have less electrons than protons
- Anions
- are ions with a negative charge
- have more electrons than protons
- Cations
A Diagram of Cations and Anions
Atomic Mass
What is the mass of an atom?
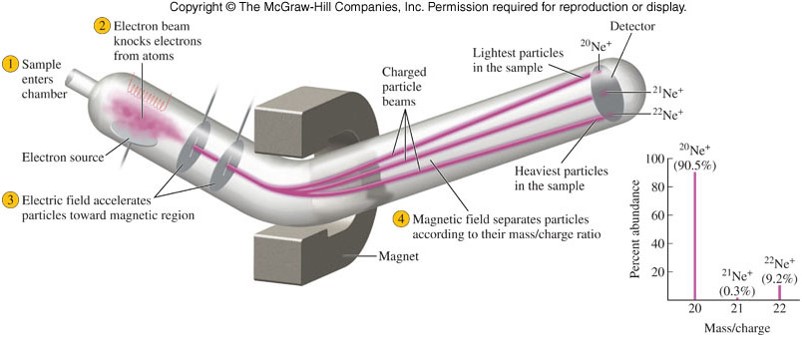
- Individual atomic masses are determined by mass spectrometry.
- Instead of expressing atomic masses in grams (a very small number), chemists express atomic masses in atomic mass units.
- An atomic mass unit (amu) is equal to 1/12 the mass of a carbon-12 atom. \[ \chem{1\, amu = \bfrac{1}{12} \times mass\, of\, {}^{12}C\, atom} \] \[ \chem{1\, amu = 1.6606 \times 10^{-24}\,g} \]
A Diagram of a Mass Spectrometer
Relative Atomic Mass
- Because most elements have multiple isomers, the masses on the periodic table cannot describe only 1 isotope's individual atomic mass.
- Therefore, the mass numbers on the periodic table are relative atomic masses:
- Relative atomic mass is the average mass of the individual isotopes of an element, taking into account the naturally occurring relative abundance of each.
- To find the relative atomic mass for an element, sum the mass contributions from each isotope of the element.
Relative Atomic Mass - Example
An unknown element (X) discovered on a planet in another galaxy was found to exist as two isotopes. Their atomic masses and percent abundances are listed in the following table. What is the relative atomic mass of the element?
| Isotope | Mass (amu) | Natural Abundance (%) |
|---|---|---|
| \( \chem{{}^{22}X} \) | 21.995 | 75.00 |
| \( \chem{{}^{20}X} \) | 19.996 | 25.00 |
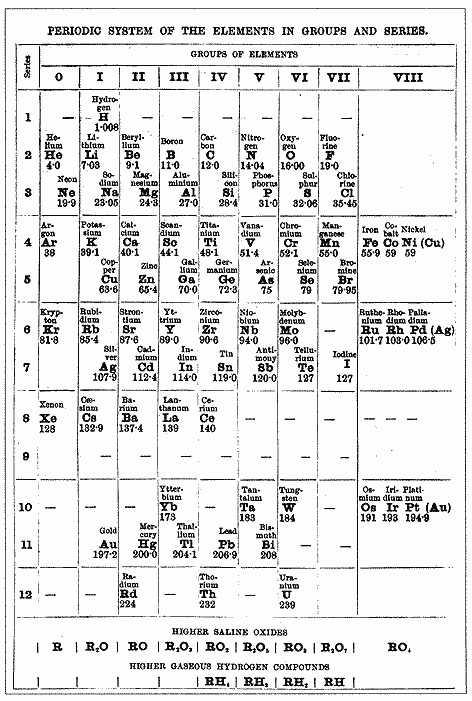
The Periodic Table
Mendeleev's Table
- Russian chemist Dmitri Mendeleev developed and published the basic arrangement of the periodic table between 1869 and 1871.
- Mendeleev arranged the elements in order of increasing relative atomic mass (protons had not been discovered yet). The elements on the modern periodic table are arranged in order of increasing atomic number.
- He also grouped elements with similar properties into columns and rows so that the properties of the elements varied in a regular pattern (periodically).
One of the first Periodic Tables
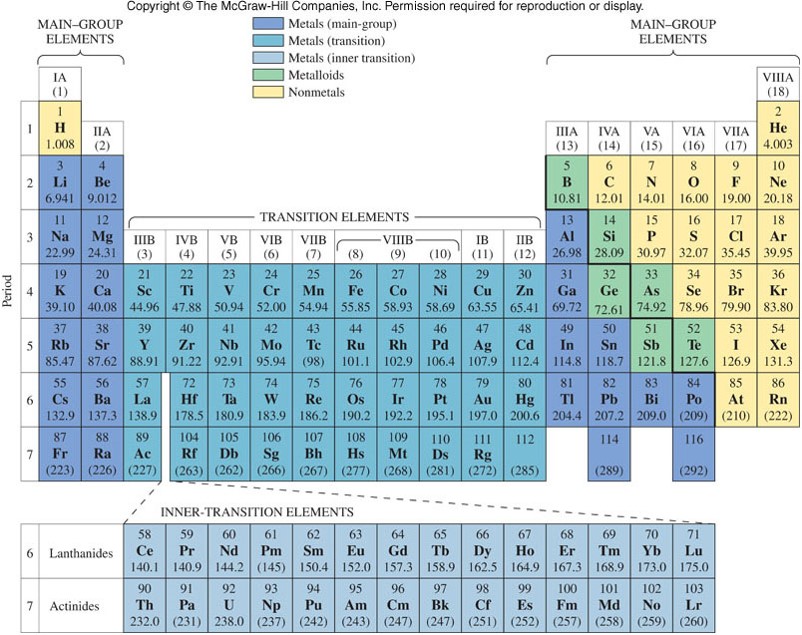
The Modern Periodic Table
- The elements in the modern periodic table are arranged by increasing atomic number (Z) and in columns and rows to emphasize periodic properties.
- The columns are collectively called families or groups and are designated in two ways:
- A Roman numeral (I through VIII) and a letter (A or B)
- An Arabic number (1-18)
- The rows are collectively called periods and are designated by an Arabic number (1-7).
Periodic Table
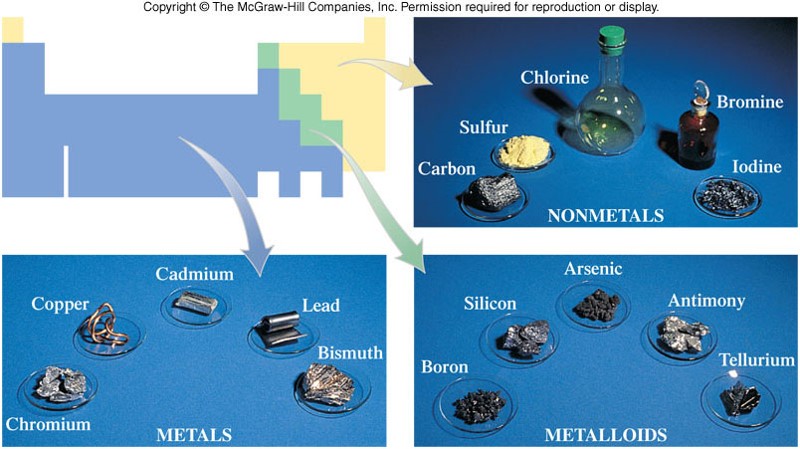
Metals, Nonmetals, and Metalloids
- The periodic table has many classifications. Groups and Periods are one classification. Another classification denotes metals, nonmetals, and metalloids.
- A stair-step line starting at boron (B) separates metals (to the left of the line) from nonmetals (to the right of the line).
- The metalloids exist along the line.
- Metalloids are elements that have physical properties resembling a metal, but the chemical reactivity of a nonmetal.
Images of Various Metals, Nonmetals, and Metalloids
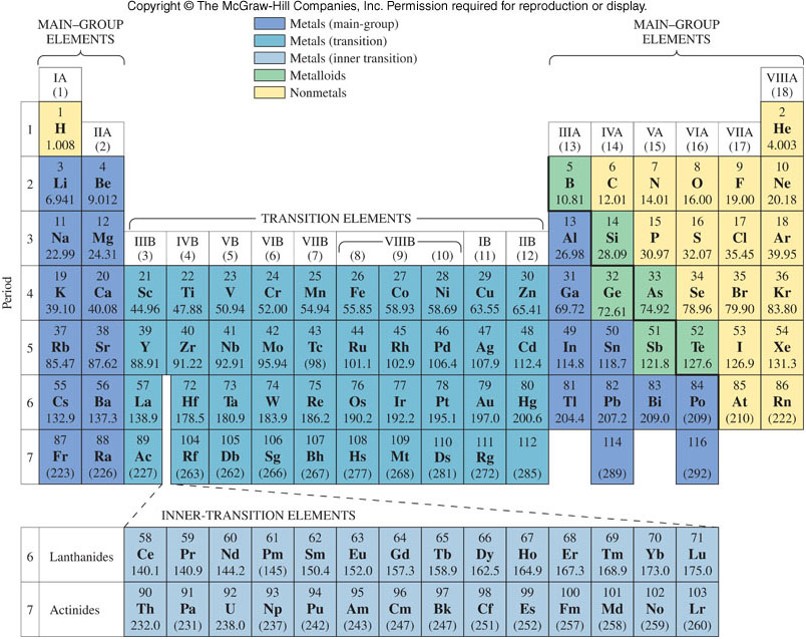
Main-Group Elements and Transition Metals
- Main-group elements (also called representative elements) contain any element in the eight groups designated with the letter A. (In the Arabic numbering, groups 1, 2, and 13-18)
- Transition metals contain any element in the 10 groups designated with the letter B. (In the Arabic numbering, groups 3-12)
- Inner-transition metals contain the lanthanides and actinides listed separately at the bottom of the table.
Periodic Table with the Main-Group Elements Colored
Common Group Names
- Some groups have descriptive names that are commonly used instead of their group numbers.
- Alkali metals
- Group 1 (IA) metals (hydrogen is a nonmetal)
- are considered reactive because the react readily with other elements and compounds
- Alkaline earth metals
- Group 2 (IIA) metals
- are more reactive than the transition metals but less reactive than alkali metals
- Alkali metals
Common Group Names - Continued
- Some groups have descriptive names that are commonly used instead of their group numbers.
- Halogens (Halides)
- Group 17 (VIIA) nonmetals
- exist naturally as diatomic molecules
- Noble gases
- Group 18 (VIIIA) nonmetals
- are also called inert gases
- are so named because they do not chemically react with other elements (with the exception of krypton and xenon)
- Halogens (Halides)
Sodium in Water

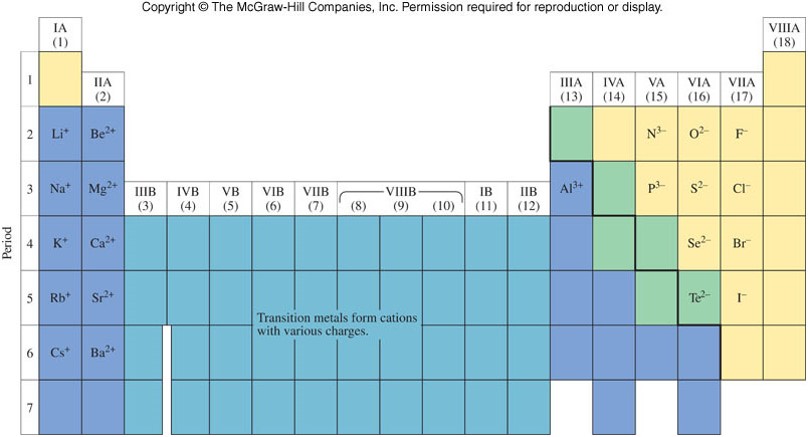
Ions and the Periodic Table
- The noble gases are the most stable (least reactive) elements on the periodic table.
- Their stability is associated with the number of electrons they contain (8 electrons in their outermost layer (or shell)).
- Many atoms in the main-group elements gain or lose electrons to achieve similar stability.
- Metals tend to lose electrons, and therefore become cations.
- Nonmetals tend to gain electrons, thereby becoming anions.
Ions on the Periodic Table
/