Redox Stability and Redox Reactions
Shaun Williams, PhD
Balancing Redox Reactions
- Write out the (unbalanced) reaction and identify the elements that are undergoing redox
- Separate the reaction into two half reactions, balancing the elements undergoing redox in each
- Balance the oxygen atoms by adding water to one side of each half reaction
- Balance the hydrogen atoms by adding \(H^+\) ions
- Balance the overall charge by adding electrons
- Combine the half reactions so that there are equal numbers of electrons on the left and right sides
- If the reaction occurs under basic conditions, add \(OH^-\) to each side to cancel out the \(H^+\)
Example 5.1
\(I^-\) is oxidized to \(IO_3^-\) by \(MnO_4^-\), which is reduced to \(Mn^{2+}\).
Example 5.2
\[ S_2O_3^{2-} + H_2O_2 \rightarrow S_4O_6^{2-} + H_2O \]
Electrochemical Potentials
- In electrochemical cells or in redox reactions that occur in solution, the thermodynamic driving force can be measured as the cell potential
- Chemical reactions are spontaneous in the direction that \(\Delta G \lt 0\), which is also the direction where the cell potential (\(E_\text{anode}-E_\text{cathode}\)) is positive
- A cell operating in the spontaneous direction is called a galvanic cell
- A cell being drive in the non-spontaeous direction is called an electrolytic cell
Example: Hydrogen Combustion
\[ 2H_2(aq)+O_2(ag) \rightleftharpoons 2H_2O(l) \;\;\; \Delta G^\circ=-237\,\frac{kJ}{mol\, H_2O} \]
- In the forward direction \[ H_2(g) \rightarrow 2H^+(aq) + 2e^- \] \[ O_2(g) + 4H^+(aq) + 4e^- \rightarrow 2 H_2O(l) \]
- Here the anode is the positive electrode and the cathode is the nagative electrode
- Under low current, the potential difference is \(1.229\, V\)
- In an electrolytic cell, the reaction is run in reverse so that anode has become positive (the cathode) and the cathode has become negative (the anode).
- In both galvanic and electrolytic cells, oxidation occurs at the anode and reduction occurs at the cathode.
Electrolysis of Water
Half-Cell Potentials
- Because electrochemical cells are broken into half-reactions, we can determine and utilize the potentials for half-reactions
- We usually take the standard hydrogen electrode as our reference (ie. we assign it a value of zero volts)
- When we construct an electrochemical cell and calculate the voltage, we simply take the difference between the half cell potentials and do not worry about the number of electrons in the reaction
Standard Reduction Potentials (or Simply Standard Potentials)
In the following table, strong oxidizing agents are reactants at the top and weak at the bottom.
Strong reducing agents are products at the bottoms and weak at the top.
| Half Reaction | Standard Potential (V) |
|---|---|
| \( F_2 + 2e^- \rightleftharpoons 2F^- \) | +2.87 |
| \( Pb^{4+} + 2e^- \rightleftharpoons Pb^{2+} \) | +1.67 |
| \( Cl_2 + 2e^- \rightleftharpoons 2Cl^- \) | +1.36 |
| \( O_2 + 4H^+ + 4e^- \rightleftharpoons 2H_2O \) | +1.23 |
| \( Ag^+ + 1e^- \rightleftharpoons Ag \) | +0.80 |
| \( Fe^{3+} + 1e^- \rightleftharpoons Fe^{2+} \) | +0.77 |
| \( Cu^{2+} + 2e^- \rightleftharpoons Cu \) | +0.34 |
| \( 2H^+ + 2e^- \rightleftharpoons H_2 \) | 0.00 |
| \( Pb^{2+}+2e^- \rightleftharpoons Pb \) | -0.13 |
| \( Fe^{2+}+2e^- \rightleftharpoons Fe \) | -0.44 |
| \( Zn^{2+}+2e^- \rightleftharpoons Zn \) | -0.76 |
| \( Al^{3+}+3e^- \rightleftharpoons Al \) | -1.66 |
| \( Mg^{2+}+2e^- \rightleftharpoons Mg \) | -2.36 |
| \( Li^+ + 1e^- \rightleftharpoons Li \) | -3.05 |
Example 5.3
Calculate the Standard Potential for the reaction in which silver ions are reduced by copper metal.
Relationship between \(E\) and \(\Delta G\)
- For systems that are in equilibrium, \(\Delta G^\circ =-nFE_{cell}^\circ \), where \(n\) is the number of moles of electrons per mole of products and \(F\) is the Faraday constant (\(\sim 96485\frac{C}{mol}\))
- This equation is for a reaction at standard conditions
- More generally, we have \(\Delta G=-nFE\), where \[ E=E^\circ - \left(\frac{RT}{nF}\right) \ln Q \] or at \(298\,K\) \[ E=E^\circ -\left(\frac{0.0592}{n}\right)\log_{10}G \]
- This is the Nernst equation
Example 5.4
For the half reaction \(2H^+ + 2e^- \rightarrow H_2\), \(E_{\frac{1}{2}}^\circ=0.000\,V\) (by definition). What is \(E_{\frac{1}{2}}\) at pH of 5 and \(P_{H_2}=1\,atm\)?
Example 5.5
Toyota fuel cell hybrid bus. The bus runs on electrical energy obtained directly from the \(H_2\)/\(O_2\) reaction. Individual fuel cells are connected in series to make a power train that charges a battery pack and drives an electric motor. Although the standard potential of the reaction is \(1.23\, V\), because of kinetic overpotentials each fuel cell in the power train operates at a voltage of about \(0.70\, V\). Despite this energy loss, the fuel cell system is still about twice as efficient as a combustion engine performing the same reaction.
What is the potential of a fuel cell (a galvanic \(H_2\)/\(O_2\) cell) operating at pH 5?
Pourbaix Diagram for Water
- Note that the value of \(E_{cell}\) does not change with pH
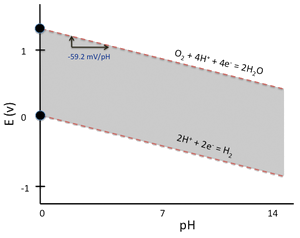
- Pourbaix diagrams are essentially electrochemical phase diagrams, which plot regions of thermodynamic stability for redox-active substances.
- The shaded area in the water Pourbaix diagram represents the conditions of potential and pH where liquid water is stable relative to hydrogen or oxygen.
- Outside the shaded region, water is thermodynamically unstable and is reduced to \(H_2(g)\) or oxidized to \(O_2(g)\)
Latimer and Frost Diagrams
- Latimer and Frost Diagrams are two other kinds of redox stability diagrams
- They contain similar information
- Latimer and Frost diagrams help predict stability relative to higher and lower oxidation states, usually at one fixed pH
- Pourbaix diagrams help understand pH-dependent equilibria
Latimer Diagram Example: Manganese in Acid
The Latimer diagram for \(Mn\) illustrates its standard reduction potentials (in \(1\, M\) acid) in oxidation states from +7 to 0.
\[ \underset{7+}{MnO_4^-} \xrightarrow{+0.564} \underset{6+}{MnO_4^{2-}} \xrightarrow{+0.274} \underset{5+}{MnO_4^{3-}} \xrightarrow{+4.27} \underset{4+}{MnO_2} \] \[ \xrightarrow{+0.95} \underset{3+}{Mn^{3+}} \xrightarrow{+1.51} \underset{2+}{Mn^{2+}} \xrightarrow{-1.18} \underset{0}{Mn} \]
Thermodynamically Stable and Unstable Oxidation States
Frost Diagrams
- Frost diagram or Frost-Ebsworth diagram is a type of graph to illustrate the relative stability of a number of different oxidation states of a particular substance.
- The graph illustrates the oxidation state vs free energy of a chemical species.
- The Frost diagram allows easier comprehension of these reduction potentials than the Latimer diagram, because the “lack of additivity of potentials” was confusing
- In a Frost diagram, we plot ΔG°⁄F (= nE°) vs. oxidation number.
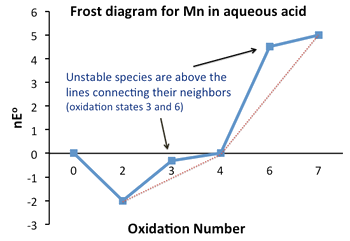
Stability
- Unstable compounds are higher on the plot than the line connecting their neighbors.
Standard Potential
- The standard potential for any electrochemical reaction is given by the slope of the line connecting the two species on a Frost diagram.

Redox Reactions with Coupled Equilibria
- Coupled equilibria (solubility, complexation, acid-base, and other reactions) change the value of \(E^\circ\), effectively by changing the concentrations of free metal ions.
- We can use the Nernst equation to calculate the value of \(E^\circ\) from the equilibrium constant for the coupled reaction.
- Alternatively, we can measure the half-cell potential with and without the coupled reaction to get the value of the equilibrium constant.
- This is one of the best ways to measure \(K_{sp}\), \(K_a\), and \(K_d\) values.
Example 5.6
Lets consider the complexation of \(Fe^{2+}\) and \(Fe^{3+}\) by \(CN^-\) ions: Which oxidation state of \(Fe\) is more strongly complexed by \(CN^-\)?
Solubility Equilibria
- The silver halides (\(AgCl\), \(AgBr\), \(AgI\)) are sparingly soluble
- We can calculate the \(K_{sp}\) of \(AgCl\) by measuring the standard potential of the \(AgCl\)/\(Ag\) couple.
- This can be done very simply by measuring the potential of a silver wire, which is in contact with solid \(AgCl\) and \(1\, M\, Cl^-(aq)\), against a hydrogen reference electrode.
- That value is then compared to the standard potential of the \(Ag^+\)/\(Ag\) couple
- Subtracting the second reaction from the first and then using \(nFE^\circ = RT\ln K\) we obtain \(K=K_{sp}=9.7\times 10^{-11}\,M^2\).
Acid-Base Equilibria
- Many electrochemical reactions involve \(H^+\) or \(OH^-\)
- For these reactions, the half-cell potentials are pH dependent
- Recall that the disproportionation reaction \( 3[MnO_4]^{2−}(aq)→2[MnO_4]^−(aq)+MnO_2(s) \) is spontaneous at \(pH=0\) (\([H^+] = 1\, M\)), from the Latimer diagram or Frost plot.
- However, when we properly balance this half reaction we see that it involves protons as a reactant
- By Le Châtelier's principle, it follows that removing protons (increasing the pH) should stabilize the reactant, \(MnO_4^{2-}\)
- Thus we would expect the +6 oxidation state of Mn, which is unstable in acid, to be stabilized in basic media
/