Coordination Chemistry and Crystal Field Theory
Shaun Williams, PhD
Coordination Compounds (Complexes)
- Coordination compounds (complexes) are molecules and extended solids that contain bonds between transition metls ions and one or more ligands
- In forming these coordinate covalent bonds, the metal ions act as Lewis acids and the ligands as Lewis bases
- Typically, the ligand has a lone pair of electrons, and the bond is formed by overlap of the molecular orbital containing this electron pair with the d-orbitals of the metal ion.
- Ligands that are commonly found in coordination complexes are neutral molecules and anions
Counting Electrons in Transition Metal Complexes
- The d-orbitals are the frontier orbitals of transition metal complexes.
- Many of the important properties of complexes - their shape, color, magnetism, and reactivity - depend on the electron occupancy of the metal's d-orbitals.
- It is important to know how to count the d-electrons
The Ferricyanide Anion
.png)
- Because transition metals are generally less electronegative than the atoms on the ligands that form the metal-ligand bond, our convention is to assign both electrons in the bond to the ligand.
- For example, in the ferricyanide complex \([Fe(CN)_6]^{3-}\), if the cyanide ligand keeps both of its electrons it is formulated as \(CN^-\).
- By difference, iron must be \(Fe^{3+}\) because the charges, \(+3 + 6(1-)\), must add up to the overall -3 charge on the complex.
How Many d-Electrons Does It Have?
- The next step is to determine how many d-electrons the \(Fe^{3+}\) ion has.
- The rule is to count all of iron's valence electrons as d-electrons. Iron is in group 8, so \[ \text{group 8}-3(\text{charge})=d^5 \;(\text{or }3d^5) \] \[ 8-3=5 \]
- The same procedure can be applied to any transition metal complex.
Example 6.1
How many d-electrons are on the metal in the following complexes:
- \([Cu(NH_3)_4]^{2+}\)
- \([Rh(OH)_3(H_2O)_3]\)
- \([Zn(NH_3)_4]^{2+}\)
- \(Ni(CO)_4\)
What Happened to the s-Orbitals?
- A frequent source of confusion about electron counting is the fate of the s-electrons on the metal.
- For example, our electron counting rules predict that \(Ti\) is \(3d^1\) in the octahedral complex \([Ti(H_2O)_6]^{3+}\).
- But the electronic configuration of a free \(Ti\) atom, according to the Aufbau principle, is \(4s^23d^2\).
- Why is the \(Ti^{3+}\) ion \(3d^1\) and not \(4s^1\)?
- The short answer is that the metal \(s\) orbitals are higher in energy in a metal complex than they are in the free atom because they have antibonding character.
Crystal Field Theory
- Crystal field theory is one of the simplest models for explaining the structures and properties of transition metal complexes.
- The theory is based on the electrostatics of the metal-ligand interaction
- Its results are only approximate in cases where the metal-ligand bond is substantially covalent.
- Because the model makes effective use of molecular symmetry, it can be surprisingly accurate in describing the magnetism, colors, structure, and relative stability of metal complexes.
Electrostatic Attraction
- Consider a positvely charged metal ion such as \(Fe^{3+}\) in the "field" of six negatively charged ligands, such as \(CN^-\).
- There are two energetic terms we need to consider.
- The first is the electrostatic attraction between the metal and ligands, which is inversely proportional to the distance between them: \[ E_{elec}=\frac{1}{4\pi \varepsilon_0}\sum_{ligands}\frac{q_Mq_L}{r_{ML}} \]
- The second term is the repulsion that arises from the Pauli exclusion principle when a third electron is added to a filled orbital.
- There is no place for this third electron to go except to a higher energy antibonding orbital.
- This is the situation when a ligand lone pair approaches an occupied metal d-orbital
Analyzing The Effect of These Electrostatic Terms
- First forming a ligand "sphere" around the metal and then moving the six ligands to the vertices of an octahedron.
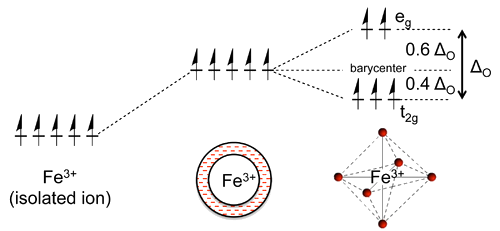
- Initially all five d-orbitals are degenerate, i.e., they have the same energy by symmetry.
- In the first step, the antibonding interaction drives up the energy of the orbitals, but they remain degenerate.
- Second step, the d-orbitals split into two symmetry classes, a lower energy, triply-degenerate set (the \(t_{2g}\) orbitals) and a higher energy, doubly degenerate set (the \(e_g\) orbitals).
What is the work done by \( 1.00\, mol \) of an ideal gas expanding reversibly from a volume of \( 22.4\, L \) to a volume of \( 44.8\, L \) at a constant temperature of \( 273\, K \)?
The Energetics

- The energy difference between the \(e_g\) and \(t_{2g}\) orbitals is given the symbol \(\Delta_O\), where the "\(O\)" stands for "octahedral."
- We will see that this splitting energy is sensitive to the degree of orbital overlap and thus depends on both the metal and the ligand.
- Relative to the midpoint energy (the barycenter), the \(t_{2g}\) orbitals are stabilized by \(\frac{2}{5}\Delta_O\) and the \(e_g\) orbitals are destabilized by \(\frac{3}{5}\Delta_O\) in an octahedral complex.
Why Does the \(d\)-Orbitals to Split into Two Sets?
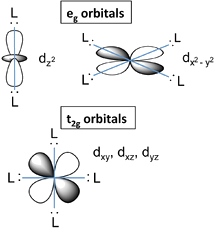
- Recall that the d-orbitals have a specific orientation with respect to the Cartesian axes.
- The lobes of the \(d_{xy}\), \(d_{xz}\), and \(d_{yz}\) orbitals (the \(t_{2g}\) orbitals) lie in the \(xy\)-, \(xz\)-, and \(yz\)-planes, respectively.
- These three \(d\)-orbitals have nodes along the \(x\)-, \(y\)-, and \(z\)-directions.
Why Does the \(d\)-Orbitals to Split into Two Sets? Continued

- The orbitals that contain the ligand lone pairs are oriented along these axes and therefore have zero overlap with the metal \(t_{2g}\) orbitals.
- These three \(d\)-orbitals must be degenerate by symmetry.
Why Does the \(d\)-Orbitals to Split into Two Sets? More

- On the other hand, the lobes of the \(d_{z^2}\) and \(d_{x^2-y^2}\) orbitals (the \(e_g\) orbitals) point directly along the bonding axes and have strong overlap with the ligand orbitals.
- While it is less intuitively obvious, these orbitals are also degenerate by symmetry and have antibonding character.
Ligand-Field Diagram for \([Ti(H_2O)_6]^{3+}\)
.png)
Spectrochemical Series
Strong and Weak Field Ligands
- The spectrochemical series ranks ligands according the energy difference \(\Delta_O\) between the \(t_{2g}\) and \(e_g\) orbitals in their octahedral complexes.
- This energy difference is measured in the spectral transition between these levels, which often lies in the visible part of the spectrum and is responsible for the colors of complexes with partially filled d-orbitals.
- Ligands that produce a large splitting are called strong field ligands, and those that produce a small splitting are called weak field ligands.
\[ \text{Weak field}\; I^- \lt Br^- \lt Cl^- \lt NO_3^- \lt F^- \lt OH^- \lt H_2O \lt \text{Pyridine} \lt NH_3 \lt NO_2^- \lt CN^- \lt CO \;\text{Strong field} \]
Orbital Overlap
- The splitting between d-electron levels reflects the antibonding interaction between the \(e_g\) metal orbitals and the ligands.
- Thus, we expect ligand field strength to correlate with metal-ligand orbital overlap.
- Ligands that bind through very electronegative atoms such as O and halogens are thus expected to be weak field, and ligands that bind through C or P are typically strong field.
- Ligands that bind through N are intermediate in strength.
- Another way to view this is within HSAB theory: hard bases tend to be weak field ligands and soft bases are strong field ligands.
Comparison of Strong and Weak Field Ligands
Energy Units
- Energy can be calculated in a number of ways and it is useful to try to relate the splitting energy \(\Delta_O\) to more familiar quantities like bond energies.
- When \(\Delta_O\) is measured optically, a photon of wavelength \(\lambda\) is absorbed as an electron is promoted from a \(t_{2g}\) to an \(e_g\) orbital.
- The photon energy is related to its wavelength and frequency by: \[ E=h\nu = \frac{hc}{\lambda}=hc\tilde{\nu} \]
- We will see that \(\Delta_O\) varies widely for transition metal complexes, from near-infrared to ultraviolet wavelengths.
- We can learn something about trends in \(\Delta_O\) by comparing a series of \(d^6\) metal complexes
Important Trends in \(\Delta_O\)
- \(Co^{3+}\) complexes have larger \(\Delta_O\) than \(Co^{2+}\) complexes with the same ligand. This reflects the electrostatic nature of the crystal field splitting.
- \(Rh^{3+}\) complexes have larger \(\Delta_O\) than \(Co^{3+}\) complexes. In general, elements in the 2nd and 3rd transition series (the 4d and 5d elements) have larger splitting than those in the 3d series.
The 4d and 5d Elements
- In comparing \(\Delta_O\) values for complexes in the 3d, 4d, and 5d series (e.g., comparing elements in the triads Co, Rh, Ir or Fe, Ru, Os), we always find \(3d \ll 4d \leq 5d\).
- This trend reflects the spatial extent of the d-orbitals and thus their overlap with ligand orbitals.
- The 3d orbitals are smaller, and they are less effective in bonding than the 4d or 5d.
- The 4d and 5d orbitals are similar to each other because of the lanthanide contraction.
- The valence orbitals of the 4d and 5d elements have similar sizes and thus the elements resemble each other in their chemistry much more than they resemble their cousins in the 3d series.
- For example, the chemistry of Ru is very similar to that of Os, but quite different from that of Fe. This is called the Lanthinide contraction.
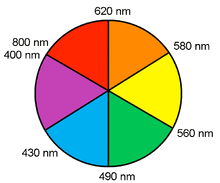
Colors of Transition Metal Complexes
- A simple, qualitative way to see the relative crystal field splitting energy, \(\Delta_O\), is to observe the color of a transition metal complex.
- The higher the energy of the absorbed photon, the larger the energy gap.
- However, the color a complex absorbs is complementary to the color it appears (i.e., the color of light it reflects), which is opposite the absorbed color on a color wheel.
\(\pi\)-Bonding Between Metals and Ligands
- An important factor that contributes to the high ligand field strength of ligands such as CO, CN-, and phosphines is \(\pi\)-bonding between the metal and the ligand.
- There are three types of pi-bonding in metal complexes:
- Back-bonding
- d-d \(\pi\) bonding
- \(\sigma\)-donor and a \(\pi\)-donor
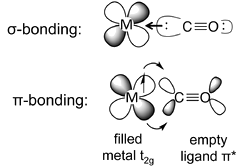
Back Bonding
- The most common situation is when a ligand such as carbon monoxide or cyanide donates its sigma (nonbonding) electrons to the metal, while accepting electron density from the metal through overlap of a metal \(t_{2g}\) orbital and a ligand \(\pi^*\) orbital.
- This situation is called "back-bonding" because the ligand donates \(\sigma\)-electron density to the metal and the metal donates \(\pi\)-electron density to the ligand.
- The ligand is thus acting as a \(\sigma\)-donor and a \(\pi\)-acceptor.
- In \(\pi\)-backbonding, the metal donates \(\pi\) electrons to the ligand \(\pi^*\) orbital, adding electron density to an antibonding molecular orbital.
- This results in weakening of the C-O bond, which is experimentally observed as lengthening of the bond (relative to free CO in the gas phase) and lowering of the C-O infrared stretching frequency.
Diagram of Backbonding
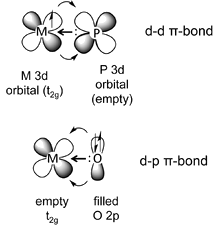
d-d \(\pi\) Bonding
- d-d \(\pi\) bonding occurs when an element such phosphorus, which has a \(\sigma\)-symmetry lone pair and an empty metal 3d orbital, binds to a metal that has electrons in a \(t_{2g}\) orbital.
- This is a common situation for phosphine complexes (e.g., triphenylphosphine) bound to low-valent, late transition metals.
- The backbonding in this case is analogous to the CO example, except that the acceptor orbital is a phosphorus 3d orbital rather than a ligand \(\pi^*\) orbital.
- Here the phosphine ligand acts as a \(\sigma\)-donor and a \(\pi\)-acceptor, forming a \(d\pi -d\pi\) bond.
\(\pi\)-Donor Ligand
- The third kind of metal-ligand \(\pi\)-bonding occurs when a \(\pi\)-donor ligand - an element with both a \(\sigma\)-symmetry electron pair and a filled orthogonal p-orbital - bonds to a metal.
- This occurs in early transition metal complexes.
- In an example, \(O^{2-}\) is acting as both a \(\sigma\)-donor and a \(\pi\)-donor.
- This interaction is typically drawn as a metal-ligand multiple bond, e.g., the \(V=O\) bond in the vanadyl cation \([VO]^{2+}\).
- Typical \(\pi\)-donor ligands are oxide (\(O^{2-}\)), nitride (\(N^{3-}\)), imide (\(RN^{2-}\)), alkoxide (\(RO^-\)), amide (\(R_2N^-\)), and fluoride (\(F^-\)).
- For late transition metals, strong \(\pi\)-donors form anti-bonding interactions with the filled d-levels, with consequences for spin state, redox potentials, and ligand exchange rates.
- \(\pi\)-donor ligands are low in the spectrochemical series.
Crystal Field Stabilization Energy, Pairing, and Hund's Rule
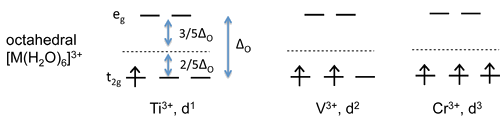
- The \(Ti^{3+}\), \(V^{3+}\), and \(Cr^{3+}\) complexes have one, two and three d-electrons respectively, which fill the degenerate \(t_{2g}\) orbitals singly.
- The spins align parallel according to Hund's rule.
- For each of these complexes we can calculate a crystal field stabilization energy, CFSE, which is the energy difference between the complex in its ground state and in a hypothetical state in which all five d-orbitals are at the energy barycenter.
Titanium, Vanadium, and Chromium

- For \(Ti^{3+}\), there is one electron stabilized by \(\frac{2}{5}\Delta_O\), so \(\text{CFSE} = (1)(\frac{2}{5})(\Delta_O) = \frac{2}{5}\Delta_O\).
- For \(V^{3+}\) \(\text{CFSE} = \frac{4}{5}\Delta_O\)
- For \(Cr^{3+}\) \(\text{CFSE} = \frac{6}{5}\Delta_O\)
A More Complicated Problem

- For \(Cr^{2+}\) complexes, which have four d-electrons, the situation is more complicated.
- Now we can have a high spin configuration \((t_{2g})^3(e_g)^1\), or a low spin configuration \((t_{2g})^4(e_g)^0\) in which two of the electrons are paired.
- What are the energies of these two states?
- High spin: \(\text{CFSE} = (3)(\frac{2}{5})\Delta_O - (1)(\frac{3}{5})\Delta_O = \frac{3}{5}\Delta_O\)
- Low spin: \(\text{CFSE} = (4)(\frac{2}{5})\Delta_O - P = \frac{8}{5}\Delta_O - P\), where \(P\) is the pairing energy
- Energy difference is \(\Delta_O-P\)
Importance of the Pairing Energy
- The pairing energy \(P\) is the energy penalty for putting two electrons in the same orbital, resulting from the electrostatic repulsion between electrons.
- For 3d elements, a typical value of \(P\) is about \(15000\, cm^{-1} \approx 667\, nm\).
- The important result here is that a complex will be low spin if \(\Delta_O \lt P\), and high spin if \(\Delta_O \lt P\).
- Because \(\Delta_O\) depends on both the metals and the ligands, it determines the spin state of the complex.
- Rules of thumb:
- 3d complexes are high spin with weak field ligands and low spin with strong field ligands.
- High valent 3d complexes (e.g., \(Co^{3+}\) complexes) tend to be low spin (large \(\Delta_O\))
- 4d and 5d complexes are always low spin (large \(\Delta_O\))
Colors and Spectra of Transition Metal Complexes
 From left: \([V(H_2O)_6]^{2+}\) (lilac), \([V(H_2O)_6]^{3+}\) (green), \([VO(H_2O)_5]^{2+}\) (blue) and \([VO(H_2O)_5]^{3+}\) (yellow).
From left: \([V(H_2O)_6]^{2+}\) (lilac), \([V(H_2O)_6]^{3+}\) (green), \([VO(H_2O)_5]^{2+}\) (blue) and \([VO(H_2O)_5]^{3+}\) (yellow).
- Transition metal complexes often have beautiful colors because, as noted above, their d-d transition energies can be in the visible part of the spectrum.
- With octahedral complexes these colors are faint (the transitions are weak) because they violate the Laporte selection rule.
- According to this rule, g -> g and u -> u transitions are forbidden in centrosymmetric complexes.
Colors of Transition Metal Complexes Continued
 From left: \([V(H_2O)_6]^{2+}\) (lilac), \([V(H_2O)_6]^{3+}\) (green), \([VO(H_2O)_5]^{2+}\) (blue) and \([VO(H_2O)_5]^{3+}\) (yellow).
From left: \([V(H_2O)_6]^{2+}\) (lilac), \([V(H_2O)_6]^{3+}\) (green), \([VO(H_2O)_5]^{2+}\) (blue) and \([VO(H_2O)_5]^{3+}\) (yellow).
- d-orbitals have g (gerade) symmetry, so d-d transitions are Laporte-forbidden.
- However octahedral complexes can absorb light when they momentarily distort away from centrosymmetry as the molecule vibrates.
- Spin flips are also forbidden in optical transitions by the spin selection rule, so the excited state will always have the same spin multiplicity as the ground state.
Non-Octahedral Complexes
- The most important non-octahedral geometries for transition metal complexes are:
- 4-coordinate: square planar and tetrahedral
- 5-coordinate: square pyramidal and trigonal bipyramidal
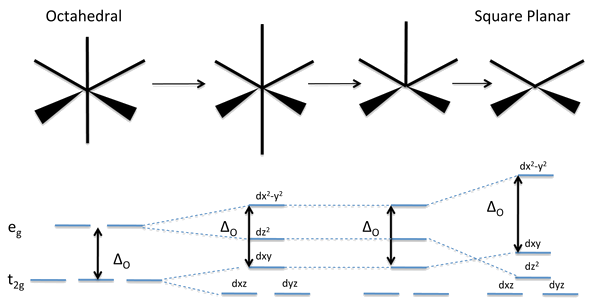
Energies of the d-Orbitals
- Energies of the d-orbitals in non-octahedral geometries.
- As we progressively distort an octahedral complex by elongating it along the z-axis (a tetragonal distortion), by removing one of its ligands to make a square pyramid, or by removing both of the ligands along the z-axis to make a square planar complex.
- In all cases, we keep the total bond order the same by making the bonds in the xy plane shorter as the bonds in the z-direction are stretched and/or broken.
- The distortion away from octahedral symmetry breaks the degeneracy of the \(t_{2g}\) and \(e_g\) orbitals.
- d-orbitals with a z-component (\(d_{xz}\), \(d_{yz}\), \(d_{z^2}\)) go down in energy as orbitals that reside in the xy plane (\(d_{xy}, d_{x^2-y^2}\)) rise in energy.
- The barycenter (the weighted average orbital energy) remains constant.
Jahn-Teller Effect
- The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that is associated with certain electron configurations.
- This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory, that orbitally degenerate molecules cannot be stable.
- The Jahn–Teller theorem essentially states that any non-linear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the molecule
Graphical Represtation of the Jahn-Teller Effect
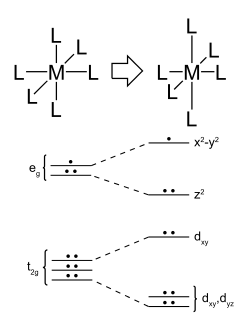
Jahn-Teller Effect: Why?
- We can understand this effect in the context of octahedral metal complexes by considering d-electron configurations in which the eg orbital set contains one or three electrons.
- The most common of these are high spin \(d^4\) (e.g., \(CrF_2\)), low spin \(d^7\) (e.g., \(NaNiO_2\)), and \(d^9\) (e.g., \(Cu^{2+}\)).
- If the complex can distort to break the symmetry, then one of the (formerly) degenerate \(e_g\) orbitals will go down in energy and the other will go up.
- More electrons will occupy the lower orbital than the upper one, resulting in an overall lowering of the electronic energy.
- A similar distortion can occur in tetrahedral complexes when the \(t_2\) orbitals are partially filled.
- Such geometric distortions that lower the electronic energy are said to be electronically driven.
Strength of the Jahn-Teller Effect
- In octahedral complexes, the Jahn–Teller effect is most pronounced when an odd number of electrons occupy the \(e_g\) orbitals.
- This situation arises in complexes with the configurations \(d^9\), low-spin \(d^7\) or high-spin \(d^4\) complexes, all of which have doubly degenerate ground states.
- In such compounds the \(e_g\) orbitals involved in the degeneracy point directly at the ligands, so distortion can result in a large energetic stabilization.
- Strictly speaking, the effect also occurs when there is a degeneracy due to the electrons in the \(t_{2g}\) orbitals (i.e. configurations such as \(d^1\) or \(d^2\), both of which are triply degenerate).
- In such cases, however, the effect is much less noticeable, because there is a much smaller lowering of repulsion on taking ligands further away from the \(t_{2g}\) orbitals, which do not point directly at the ligands.
Expected Strength of Jahn-Teller Effect
In the following table - w: weak Jahn–Teller effect (\(t_{2g}\) orbitals unevenly occupied), s: strong Jahn–Teller effect expected (\(e_g\) orbitals unevenly occupied), blank: no Jahn–Teller effect expected.
| Number of d electrons |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High/Low Spin | HS | LS | HS | LS | HS | LS | HS | LS | ||||||
| Strength of J-T Effect |
w | w | s | w | w | w | w | s | s | |||||
Tetrahedral Complexes
- Tetrahedral complexes are formed with late transition metal ions (\(Co^{2+}, Cu^{2+}, Zn^{2+}, Cd^{2+}\)) and some early transition metals (\(Ti^{4+}, Mn^{2+}\)), especially in situations where the ligands are large.
- In these cases the small metal ion cannot easily accomodate a coordination number higher than four.
- Examples of tetrahedal ions and molecules are \([CoCl_4]^{2-}\), \([MnCl_4]^{2-}\), and \(TiX_4\) (\(X\) = halogen).
- Tetrahedral coordination is also observed in some oxo-anions such as \([FeO_4]^{4-}\), which exists as discrete anions in the salts \(Na_4FeO_4\) and \(Sr_2FeO_4\), and in the neutral oxides \(RuO_4\) and \(OsO_4\).
- The metal carbonyl complexes \(Ni(CO)_4\) and \([Co(CO)_4]^-\) are also tetrahedral.
Splitting the d-Orbitals
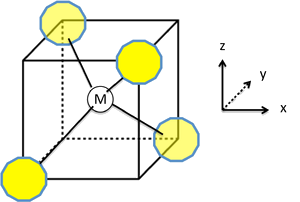
- The splitting of the d-orbitals in a tetrahedral crystal field can be understood by connecting the vertices of a tetrahedron to form a cube
- The tetrahedral M-L bonds lie along the body diagonals of the cube.
- The \(d_{z^2}\) and \(d_{x^2-y^2}\) orbitals point along the cartesian axes, i.e., towards the faces of the cube, and have the least contact with the ligand lone pairs.
Splitting the d-Orbitals Continued

- Therefore these two orbitals form a low energy, doubly degenerate \(e\) set.
- The \(d_{xy}\), \(d_{yz}\), and \(d_{xz}\) orbitals point at the edges of the cube and form a triply degenerate \(t_2\) set.
- While the \(t_2\) orbitals have more overlap with the ligand orbitals than the \(e\) set, they are still weakly interacting compared to the \(e_g\) orbitals of an octahedral complex.
Splitting the d-Orbitals Continued

- Tetrahedral complexes often have vibrant colors because they lack the center of symmetry that forbids a d-d* transition.
- Because the low energy transition is allowed, these complexes typically absorb in the visible range and have extinction coefficients that are 1-2 orders of magnitude higher than the those of the corresponding octahedral complexes.
Stability of Transition Metal Complexes
- The crystal field stabilization energy (CFSE) is an important factor in the stability of transition metal complexes.
- Complexes with high CFSE tend to be thermodynamically stable (i.e., they have high values of \(K_a\), the equilibrium constant for metal-ligand association) and are also kinetically stable.
- They are kinetically stable because ligand substitution requires that they dissociate (lose a ligand), associate (gain a ligand), or interchange (gain and lose ligands at the same time) in the transition state.
- These distortions in coordination geometry lead to a large activation energy if the CFSE is large, even if the product of the ligand exchange reaction is also a stable complex.
Other Stabilization Factors
- There are two other important factors that contribute to complex stability:
- Hard-soft interactions of metals and ligands (which relate to the energy of complex formation)
- The chelate effect, which is an entropic contributor to complex stability.
Hard-Soft Interactions: Hard
- Hard acids are typically small, high charge density cations that are weakly polarizable such as \(H^+, Li^+, Na^+, Be^{2+}, Mg^{2+}, Al^{3+}, Ti^{4+}, Cr^{6+}\).
- Electropositive metals in high oxidation states are typically hard acids.
- These elements are predominantly found in oxide minerals, because \(O^{2-}\) is a hard base.
- Some hard bases include \(H_2O, OH^-, O^{2-}, F^-, NO_3^-, Cl^-, NH_3\).
- The hard acid-base interaction is primarily electrostatic. Complexes of hard acids with hard bases are stable because of the electrostatic component of the CFSE.
Hard-Soft Interactions: Soft
- Soft acids are large, polarizable, electronegative metal ions in low oxidation states such as \(Ni^0, Hg^{2+}, Cd^{2+}, Cu^+, Ag^+, Au^+\).
- Soft bases are anions/neutral bases such as \(H^-, C_2H_4, CO, PR_3, R_2S, CN^-\).
- Soft acids typically occur in nature as sulfide or arsenide minerals.
- The bonding between soft acids and soft bases is predominantly covalent.
- For example, metal carbonyls bind through a covalent interaction between a zero- or low-valent metal and neutral CO to form \(Ni(CO)_4, Fe(CO)_5, Co(CO)_4^-, Mn_2(CO)_{10}, W(CO)_6\), and related compounds.
Like Binds Like
-
The preference for hard-hard and soft-soft interactions ("like binds like") is nicely illustrated in the properties of the copper halides:
\[ \underbrace{CuF}_\text{unstable} \;\;\; \underbrace{CuI}_\text{stable} \]
\[ \underbrace{CuF_2}_\text{stable} \;\;\; \underbrace{CuI_2}_\text{unstable} \]
Chelate and Macrocyclic Effects
- Ligands that contain more than one binding site for a metal ion are called chelating ligands (from the Greek word χηλή, chēlē, meaning "claw").
- As the name implies, chelating ligands have high affinity for metal ions relative to ligands with only one binding group (which are called monodentate = "single tooth") ligands.
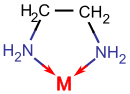
Consider Ethylenediamine
- Consider the two complexation equilibria in aqueous solution \[ [Co(H_2O)_6]^{2+} + 6 NH_3 \rightleftharpoons [Co(NH_3)_6]^{2+} + 6 H_2O \] \[ [Co(H_2O)_6]^{2+} + 3 en \rightleftharpoons [Co(en)_3]^{2+} + 6 H_2O \]
- Electronically, the ammonia and en ligands are very similar, since both bind through N and since the Lewis base strengths of their nitrogen atoms are similar.
- This means that \(\Delta H^\circ\) must be very similar for the two reactions, since six Co-N bonds are formed in each case.
- Interestingly however, we observe that the equilibrium constant is 100,000 times larger for the second reaction than it is for the first.
Consider Ethylenediamine: Why?
\[ [Co(H_2O)_6]^{2+} + 6 NH_3 \rightleftharpoons [Co(NH_3)_6]^{2+} + 6 H_2O \] \[ [Co(H_2O)_6]^{2+} + 3 en \rightleftharpoons [Co(en)_3]^{2+} + 6 H_2O \]
- The big difference between these two reactions is that the second one involves "condensation" of fewer particles to make the complex.
- This means that the entropy changes for the two reactions are different.
- The first reaction has a \(\Delta S^\circ\) value close to zero, because their is the same number of molecules on both sides of the equation.
- The second one has a positive \(\Delta S^\circ\) because four molecules come together but seven molecules are produced.
- The difference between them (\(\Delta \Delta S^\circ\)) is about \(+100 \frac{J}{mol\, K}\).
The Chelating Effect
- The bottom line is that the chelate effect is entropy-driven.
- It follows that the more binding groups a ligand contains, the more positive the \(\Delta S^\circ\) and the higher the \(K_f\) will be for complex formation.
- In this regard, the hexadentate ligand ethylenediamine tetraacetic acid (EDTA) is an optimal ligand for making octahedral complexes because it has six binding groups.
- In basic solutions where all four of the COOH groups are deprotonated, the chelate effect of the \(\text{EDTA}^{4-}\) ligand is approximately \(10^{15}\).
- This means, for a given metal ion, \(K_f\) is \(10^{15}\) times larger for \(\text{EDTA}^{4-}\) than it would be for the relevant monodentate ligands at the same concentration.
- \(\text{EDTA}^{4-}\) tightly binds essentially any 2+, 3+, or 4+ ion in the periodic table, and is a very useful ligand for both analytical applications and separations.
Ethylenediaminetetraacetic acid (EDTA)
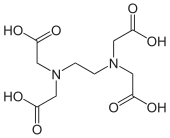
The Macrocyclic Effect
- The macrocyclic effect follows the same principle as the chelate effect, but the effect is further enhanced by the cyclic conformation of the ligand.
- Macrocyclic ligands are not only multi-dentate, but because they are covalently constrained to their cyclic form, they allow less conformational freedom.
- The ligand is said to be "pre-organized" for binding, and there is little entropy penalty for wrapping it around the metal ion.
- For example heme b is a tetradentate cyclic ligand which strongly complexes transition metal ions, including (in biological systems) \(Fe^{2+}\).
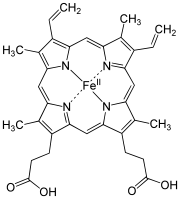
/