Chapter 18
Representative Metals, Metalloids, and Nonmetals
Shaun Williams, PhD
Reviewing the Periodic Table Regions
- Representative elements:
- Groups 1A – 8A (filling s and p orbitals)
- Transition metals:
- Center of the table (filling d orbitals)
- Lanthanides and Actinides:
- Listed separately, on the bottom of the table (filling 4f and 5f orbitals)
- Metalloids:
- Separate metals from nonmetals
The Atomic Radii of Some Representative Elements (in Picometers)
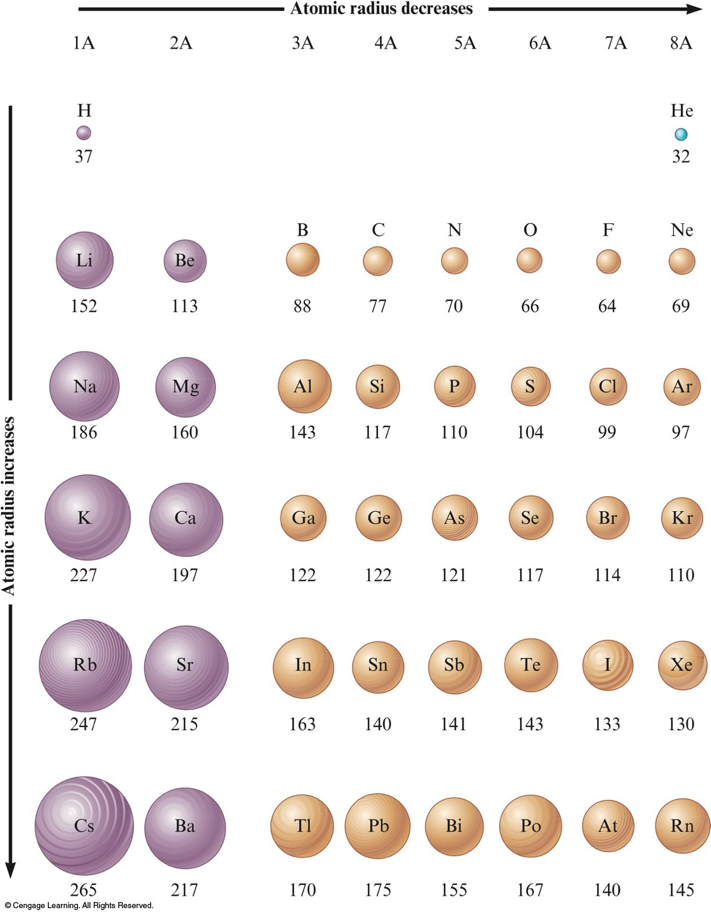
Concept Check
Which should be the larger atom, Na or Cl? Why?
Na
Concept Check
Which should be the larger atom, Li or Cs? Why?
Cs
Distribution (Mass Percent) of the 18 Most Abundant Element on Earth
| Element | Mass Percent | Element | Mass Percent |
|---|---|---|---|
| Oxygen | 49.2 | Chlorine | 0.19 |
| Silicon | 25.7 | Phosphorous | 0.11 |
| Aluminum | 7.50 | Manganese | 0.09 |
| Iron | 4.71 | Carbon | 0.08 |
| Calcium | 3.39 | Sulfur | 0.06 |
| Sodium | 2.63 | Barium | 0.04 |
| Potassium | 2.40 | Nitrogen | 0.03 |
| Magnesium | 1.93 | Fluorine | 0.03 |
| Hydrogen | 0.87 | All others | 0.49 |
| Titanium | 0.58 |
Abundance of the Elements in the Human Body
| Major Element | Mass Percent | Trace Elements (in alphabetical order) |
|---|---|---|
| Oxygen | 65.0 | Arsenic |
| Carbon | 18.0 | Chromium |
| Hydrogen | 10.0 | Cobalt |
| Nitrogen | 3.0 | Copper |
| Calcium | 1.4 | Fluorine |
| Phosphorus | 1.0 | Iodine |
| Magnesium | 0.50 | Manganese |
| Potassium | 0.34 | Molybdenum |
| Sulfur | 0.26 | Nickel |
| Sodium | 0.14 | Selenium |
| Chlorine | 0.14 | Silicon |
| Iron | 0.004 | Vanadium |
| Zinc | 0.003 |
Alkali Metals: Sources and Methods of Preparation
| Element | Source | Method of Preparation |
|---|---|---|
| Lithium | Silicate minerals such as spodumene, \( \chem{LiAl(Si_2O_6)} \) | Electrolysis of molten \( \chem{LiCl} \) |
| Sodium | \( \chem{NaCl} \) | Electrolysis of molten \( \chem{NaCl} \) |
| Potassium | \( \chem{KCl} \) | Electrolysis of molten \( \chem{KCl} \) |
| Rubidium | Impurity in lepidolite, \( \chem{Li_2(F, OH)_2Al_2(SiO_3)_3} \) | Reduction of \(\chem{RbOH}\) with \(\chem{Mg}\) and \(\chem{H_2}\) |
| Cesium | Pollucite(\( \chem{Cs_4Al_4Si_9O_26\cdot H_2O} \)) and an impurity if lepidolite | Reduction of \(\chem{CsOH}\) with \(\chem{Mg}\) and \(\chem{H_2}\) |
Selected Reactions of the Alkali Metals
| Reaction | Comment |
|---|---|
| \(\chem{2M+X_2 \rightarrow 2MX}\) | \(\chem{X_2}\) is any halogen molecule |
| \(\chem{4Li+O_2 \rightarrow 2Li_2O}\) | Excess oxygen |
| \(\chem{2Na+O_2 \rightarrow Na_2O_2}\) | |
| \(\chem{M+O_2 \rightarrow MO_2}\) | \(\chem{M}\) is \(\chem{K}\), \(\chem{Rb}\), or \(\chem{Cs}\) |
| \(\chem{2M+S \rightarrow M_2S}\) | |
| \(\chem{6Li+N_2 \rightarrow 2Li_3N}\) | \(\chem{Li}\) only |
| \(\chem{12M+P_4 \rightarrow 4M_3P}\) | |
| \(\chem{2M+H_2 \rightarrow 2MH}\) | |
| \(\chem{2M+2H_2O \rightarrow 2MOH+H_2}\) | |
| \(\chem{2M+2H^+ \rightarrow 2M^++H_2}\) | Violent reaction! |
Exercise
Predict the products formed by the following reactants: \(\chem{Na_2O_2(s)+H_2O(l)}\)
\(\chem{Na_2O_2(s)+H_2O(l)\rightarrow NaOH(aq)+H_2O_2(aq)}\)
Hydrides
- Binary compounds containing hydrogen:
- Ionic hydrides: hydrogen + the most active metals (eg; \(\chem{LiH}\), \(\chem{CaH_2}\))
- Covalent hydrides: hydrogen + other nonmetals (eg; \(\chem{H_2O}\), \(\chem{CH_4}\), \(\chem{NH_3}\))
- Metallic (interstitial) hydrides: transition metal crystals treated with \(\chem{H_2}\) gas
Exercise
Predict the products formed by the following reactants: \(\chem{LiH(s)+H_2O(l)}\)
\(\chem{LiH(s)+H_2O(l)\rightarrow H_2(g)+LiOH(aq)}\)
Alkaline Earth Metals
- Very reactive
- Great practical importance:
- Human life (\(\chem{Ca}\) and \(\chem{Mg}\))
Selected Reactions of the Group 2A Elements
| Reaction | Comment |
|---|---|
| \(\chem{M+X_2 \rightarrow MX_2}\) | \(\chem{X_2}\) is any halogen molecule |
| \(\chem{2M+O_2 \rightarrow 2MO}\) | \(\chem{Ba}\) gives \(\chem{BaO_2}\) as well |
| \(\chem{M+S \rightarrow MS}\) | |
| \(\chem{3M+N_2 \rightarrow M_3N_2}\) | High temperatures |
| \(\chem{6M+P_4 \rightarrow 2M_3P_2}\) | High temperatures |
| \(\chem{M+H_2 \rightarrow MH_2}\) | \(\chem{M}\) is \(\chem{Ca}\), \(\chem{Sr}\), or \(\chem{Ba}\); high temperatures; \(\chem{Mg}\) at high pressure |
| \(\chem{M+2H_2O \rightarrow M(OH)_2+H_2}\) | \(\chem{M}\) is \(\chem{Ca}\), \(\chem{Sr}\), or \(\chem{Ba}\) |
| \(\chem{M+2H^+ \rightarrow M^{2+}+H_2}\) | |
| \(\chem{Be+2OH^-+2H_2O \rightarrow Be(OH)_2^{2-}+H_2}\) |
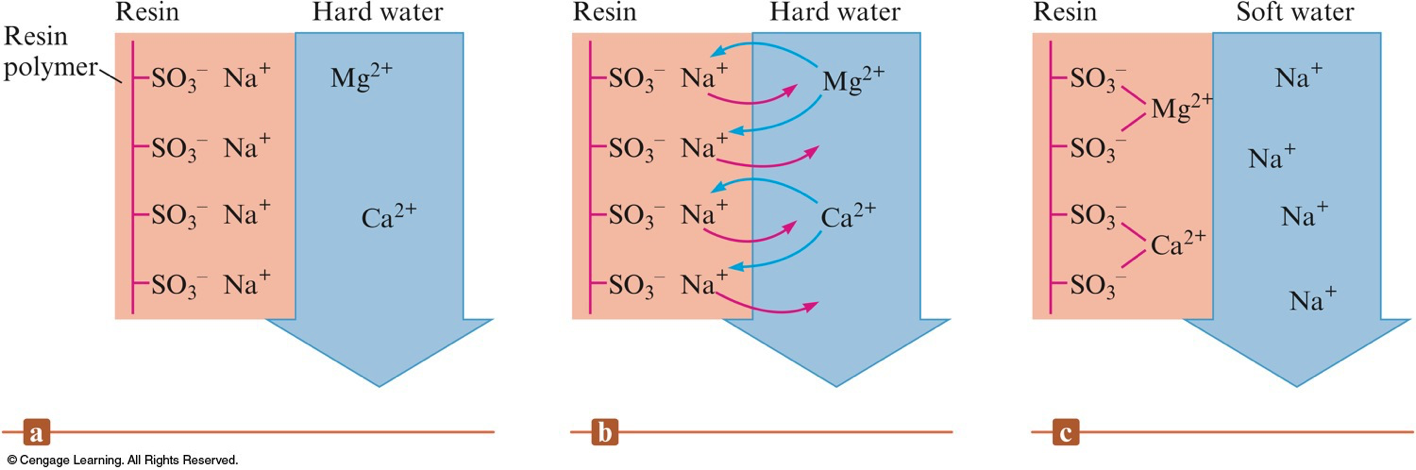
Ion Exchange
- \(\chem{Ca^{2+}}\) and \(\chem{Mg^{2+}}\) are often removed during ion exchange, releasing \(\chem{Na^+}\) into solution.
- Ion exchange resin – large molecules that have many ionic sites.
A Schematic Representation of a Typcical Cation Exchange Resin
Group 3A Elements
- Group 3A elements generally show the increase in metallic character in going down the group that is characteristic of the representative elements.
- \(\chem{B}\), \(\chem{Al}\), \(\chem{Ga}\), \(\chem{In}\), \(\chem{Tl}\)
Some Physical Properties, Sources, and Methods of Preparation of the Group 3A Elements
| Element | Radius of \(\chem{M^{3+}}\) (pm) |
Ionization Energy (kJ/mol) |
\(\mathcal{E}^\circ\,(\mathrm{V})\) \(\chem{M^{3+}+3e^-\rightarrow M}\) |
Source | Method of Preparation |
|---|---|---|---|---|---|
| Boron | 20 | 798 | - | Kernite, a form of borax (\(\chem{Na_2B_4O_7\cdot 4H_2O}\)) | Reduction by \(\chem{Mg}\) or \(\chem{H_2}\) |
| Aluminum | 50 | 581 | -1.66 | Bauxite (\(\chem{Al_2O_3}\)) | Electrolysis of \(\chem{Al_2O_3}\) in molten \(\chem{Na_3AlF_6}\) |
| Gallium | 62 | 577 | -0.53 | Traces in various minerals | Reduction with \(\chem{H_2}\) or electrolysis |
| Indium | 81 | 556 | -0.34 | Traces in various minerals | Reduction with \(\chem{H_2}\) or electrolysis |
| Thallium | 95 | 589 | 0.72 | Traces in various minerals | Electrolysis |
Selected Reactions of the Group 3A Elements
| Reaction | Comment |
|---|---|
| \(\chem{2M+3X_2 \rightarrow 2MX_3}\) | \(\chem{X_2}\) is any halogen molecule; \(\chem{Tl}\) gives \(\chem{TlX}\) as well, but no \(\chem{TlI_3}\) |
| \(\chem{4M+3O_2 \rightarrow 2M_2O_3}\) | High temperatures; \(\chem{Tl}\) gives \(\chem{Tl_2O}\) as well |
| \(\chem{2M+3S \rightarrow M_2S_3}\) | High temperatures; \(\chem{Tl}\) gives \(\chem{Tl_2S}\) as well |
| \(\chem{2M+N_2 \rightarrow 2MN}\) | \(\chem{M}\) is \(\chem{Al}\) only |
| \(\chem{2M+6H^+ \rightarrow 2M^{3+}+3H_2}\) | \(\chem{M}\) is \(\chem{Al}\), \(\chem{Ga}\), or \(\chem{In}\); \(\chem{Tl}\) gives \(\chem{Tl^+}\) |
| \(\chem{2M+2OH^-+6H_2O \rightarrow 2M(OH)_4^-+3H_2}\) | \(\chem{M}\) is \(\chem{Al}\) or \(\chem{Ga}\) |
Group 4A Elements
- Contains two of the most important elements on earth: carbon and silicon.
- Can form four covalent bonds to nonmetals.
- \(\chem{CH_4}\), \(\chem{SiF_4}\), \(\chem{GeBr_4}\)
Some Physical Properties, Sources, and Methods of Preparation of the Group 4A Elements
| Element | Electronegativity | Melting Point (\({}^\circ \mathrm{C}\)) | Boiling Point (\({}^\circ \mathrm{C}\)) | Source | Method of Preparation |
|---|---|---|---|---|---|
| Carbon | 2.6 | 3727 (sublimes) | - | Graphite, diamond, petroleum, coal | - |
| Silicon | 1.9 | 1410 | 2355 | Silicate minerals, silica | Reduction of \(\chem{K_2SiF_6}\) with \(\chem{Al}\), or reduction of \(\chem{SiO_2}\) with \(\chem{Mg}\) |
| Germanium | 2.0 | 937 | 2830 | Germinate (mixture of copper, iron, and germanium sulfides) | Reduction of \(\chem{GeO_2}\) with \(\chem{H_2}\) or \(\chem{C}\) |
| Tin | 2.0 | 327 | 2270 | Cassiterite (\(\chem{SnO_2}\)) | Reduction of \(\chem{SnO_2}\) with \(\chem{C}\) |
| Lead | 2.3 | 327 | 1740 | Galena (\(\chem{PbS}\)) | Reduction of \(\chem{PbS}\) with \(\chem{O_2}\) to form \(\chem{PbO_2}\) and then reduction with \(\chem{C}\) |
Selected Reactions of the Group 4A Elements
| Reaction | Comment |
|---|---|
| \(\chem{M+2X_2 \rightarrow MX_4}\) | \(\chem{X_2}\) is any halogen molecule; \(\chem{M}\) is \(\chem{Ge}\) or \(\chem{Sn}\); \(\chem{Pb}\) gives \(\chem{PbX_2}\) |
| \(\chem{M+O_2 \rightarrow MO_2}\) | \(\chem{M}\) is \(\chem{Ge}\) or \(\chem{Sn}\); high temperatures; \(\chem{Pb}\) gives \(\chem{PbO}\) or \(\chem{Pb_3O_4}\) |
| \(\chem{M+2H^+ \rightarrow M^{2+}+H_2}\) | \(\chem{M}\) is \(\chem{Sn}\) or \(\chem{Pb}\) |
Group 5A Elements
- Exhibits varied chemical properties.
- \(\chem{N}\), \(\chem{P}\), \(\chem{As}\), \(\chem{Sb}\), \(\chem{Bi}\)
Some Physical Properties, Sources, and Methods of Preparation of the Group 5A Elements
| Element | Electronegativity | Source | Method of Preparation |
|---|---|---|---|
| Nitrogen | 3.0 | Air | Liquifaction of Air |
| Phosphorus | 2.2 | Phosphate rock (\(\chem{Ca_3(PO_4)_2}\)), fluorapatite (\(\chem{Ca_5(PO_4)_3F}\)) | \(\chem{2Ca_3(PO_4)_2+6SiO_2 \rightarrow 6CaSiO_3+P_4O_{10}}\) \(\chem{P_4O_{10}+10C\rightarrow 4P+10CO}\) |
| Arsenic | 2.2 | Arsenopyrite (\(\chem{Fe_3As_2}\), \(\chem{FeS}\)) | Heating arsenopyrite in the absence of air |
| Antimony | 2.1 | Stibnite (\(\chem{Sb_2S_3}\)) | Roasting \(\chem{Sb_2S_3}\) in air to form \(\chem{Sb_2O_3}\) and then reduction with carbon |
| Bismuth | 2.0 | Bismite (\(\chem{Bi_2O_3}\)), bismuth glance (\(\chem{Bi_2S_3}\)) | Roasting \(\chem{Bi_2S_3}\) in air to form \(\chem{Bi_2O_3}\) and then reduction with carbon |
Nitrogen
- The great stability of the \(\chem{N\equiv N}\) bond means that most binary compounds containing nitrogen decompose exothermically to the elements. $$ \begin{align} \chem{NO_2(g)} \rightarrow & \chem{\frac{1}{2}N_2(g)+O_2(g)} \\ & \Delta H^\circ = -34\,\mathrm{kJ} \\ \chem{N_2H_4(a)} \rightarrow & \chem{N_2(g)+2H_2(g)} \\ & \Delta H^\circ = -95\,\mathrm{kJ} \end{align} $$
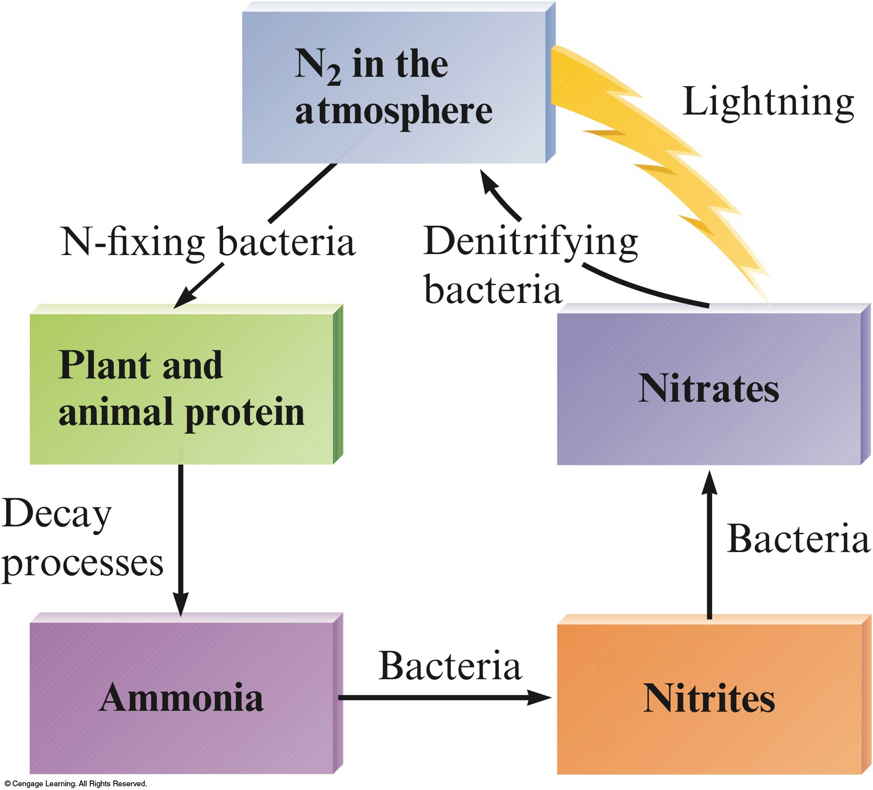
Nitrogen Fixation
- The process of transforming N2 to other nitrogen–containing compounds.
- The Haber Process: $$ \begin{align} \chem{N_2(g)+3H_2(g)} \rightarrow & \chem{2NH_3(g)} \\ & \Delta H^\circ = -92\,\mathrm{kJ} \end{align} $$
The Nitrogen Cycle
Nitrogen Hydrides
- Ammonia, \(\chem{NH_3}\)
- Fertilizers
- Hydrazine, \(\chem{N_2H_4}\)
- Rocket propellant, manufacture of plastics, agricultural pesticides
- Monomethylhydrazine, \(\chem{N_2H_3(CH_3)}\)
- Rocket fuels
Nitrogen Oxides
- Nitrogen in its oxides has oxidation states from +1 to +5.
- In other compounds, nitrogen could have oxidation states of -1 to -3.
| Compound | Oxidation State of \(\chem{N}\) |
|---|---|
| \(\chem{N_2O}\) | +1 |
| \(\chem{NO}\) | +2 |
| \(\chem{N_2O_3}\) | +3 |
| \(\chem{NO_2}\) | +4 |
| \(\chem{HNO_3}\) | +5 |
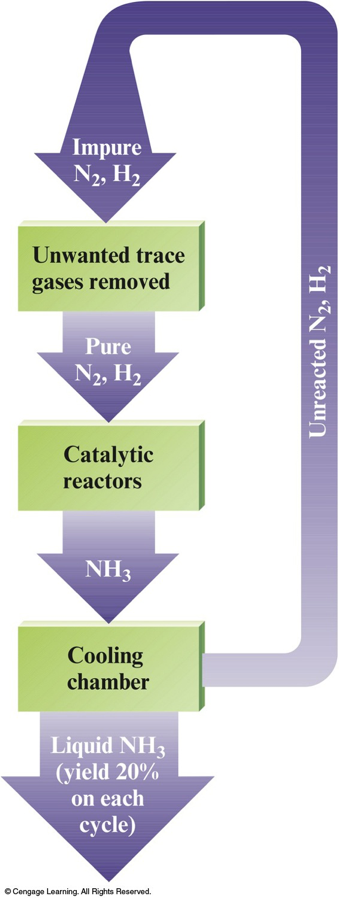
Nitrogen Oxyacids
- Nitric acid, \(\chem{HNO_3}\) $$ \chem{4HNO_3(l)} \xrightarrow{h\nu} \chem{4NO_2(g)+2H_2O(l)+O_2(g)} $$
- Nitrous acid, \(\chem{HNO_2}\) $$ \chem{HNO_2(aq) \rightleftharpoons H^+(aq)+NO_2^-(aq)} $$
The Ostwald Process
Allotropes of Phosphorus
- White Phosphorus = \(\chem{P_4}\) (tetrahedral) - very reactive
- Black Phosphorus = crystalline structure - much less reactive
- Red Phosphorus = amorphous with \(\chem{P_4}\) chains $$ \begin{align} \chem{P(white)} & \xrightarrow{\text{heat, 1 atm, no air}} \chem{P(red)} \\ \chem{P(white)\;\mathrm{or}\;P(red)} & \xrightarrow{\text{high pressure}} \chem{P(black)} \\ \end{align} $$
Allotropes of Phosphorus
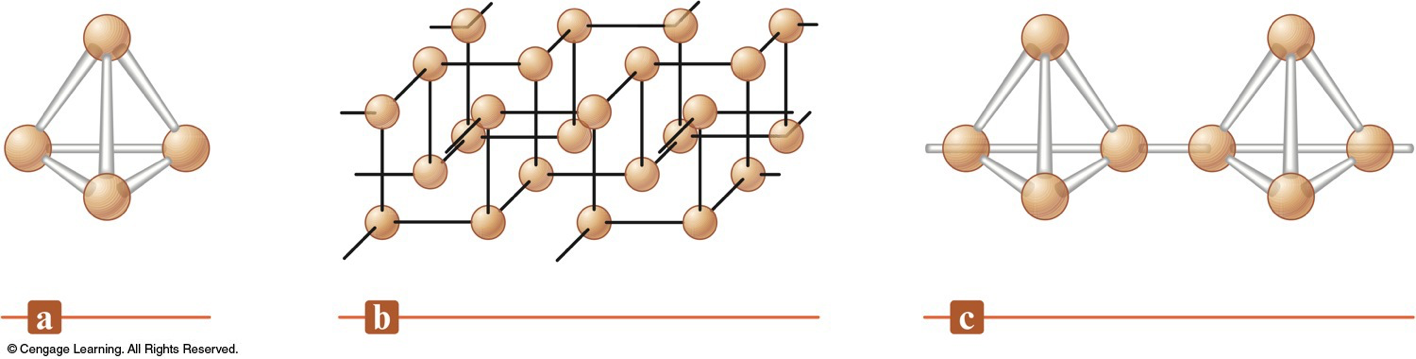
- \(\chem{P_{white}}\)
- \(\chem{P_{black}}\)
- \(\chem{P_{red}}\)
Phosphorus Oxyacids
- Phosphoric acid, \(\chem{H_3PO_4}\)
- Phosphorous acid, \(\chem{H_3PO_3}\)
- Hypophosphorous acid, \(\chem{H_3PO_2}\)
The Group 6A Elements
- \(\chem{O}\), \(\chem{S}\), \(\chem{Se}\), \(\chem{Te}\), \(\chem{Po}\)
- Although in Group 6A there is the usual tendency for metallic properties to increase going down the group, none of the Group 6A elements behaves as a typical metal.
- Can form covalent bonds with other nonmetals.
Some Physical Properties, Sources, and Methods of Preparation of the Group 6A Elements
| Element | Electronegativity | Radius of \(\chem{X^{2-}}\) (pm) | Source | Method of Preparation |
|---|---|---|---|---|
| Oxygen | 3.4 | 140 | Air | Distillaton from liquid air |
| Sulfur | 2.6 | 184 | Sulfur deposits | Melted with hot water and pumped to the surface |
| Selenium | 2.6 | 198 | Impurity in sulfide ores | Reduction of \(\chem{H_2SeO_4}\) with \(\chem{SO_2}\) |
| Tellurium | 2.1 | 221 | Nagyagite (mixed sulfide and telluride) | Reduction of ore with \(\chem{SO_3}\) |
| Polonium | 2.0 | 230 | Pitchblende |
Oxygen
- \(\chem{O_2}\) makes up 21% of the Earth’s atmosphere.
- \(\chem{O_3}\) (ozone) exists naturally in the upper atmosphere of the Earth.
- Ozone layer absorbs UV light and acts as a screen to prevent this radiation from penetrating to the Earth’s surface.
- Scientists have become concerned that Freons and nitrogen dioxide are promoting the destruction of the ozone layer.
Ozone
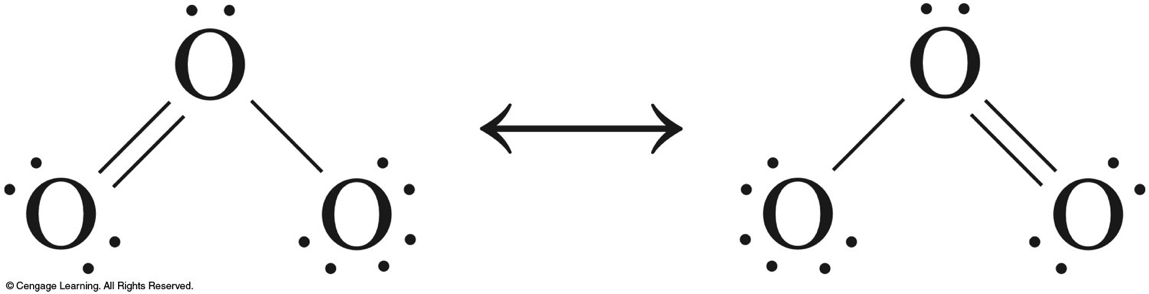
$$ \begin{align} & \chem{3O_2(g) \rightleftharpoons 2O_3(g)\;\;\;\;K\approx 10^{-56}} \\ & \chem{O_3} \xrightarrow{h\nu} \chem{O_2+O} \end{align} $$
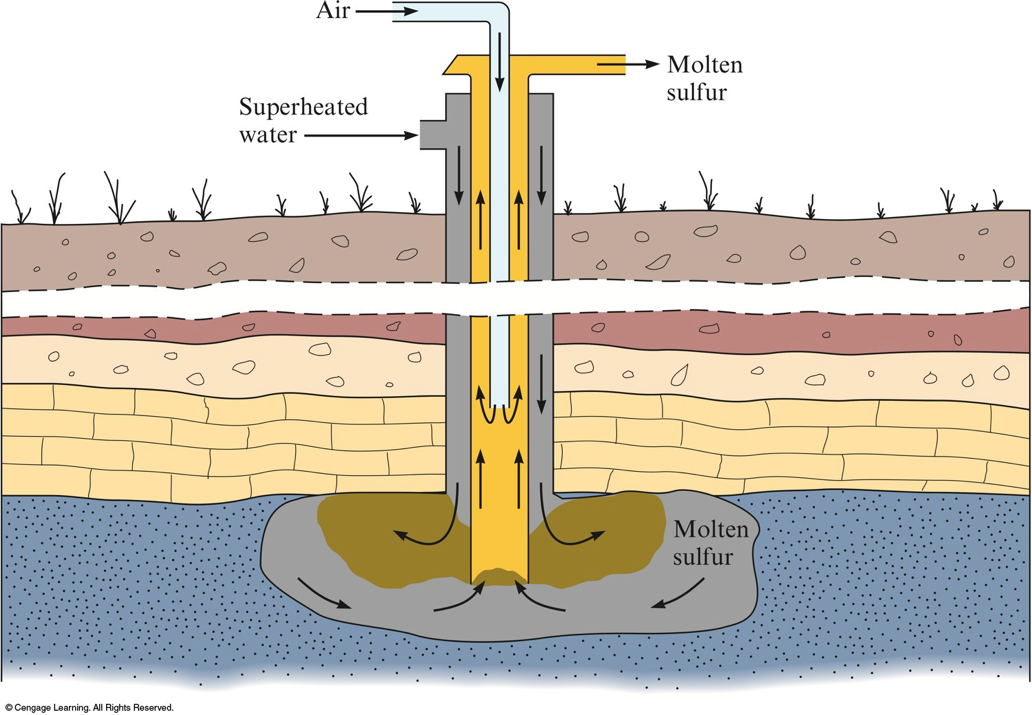
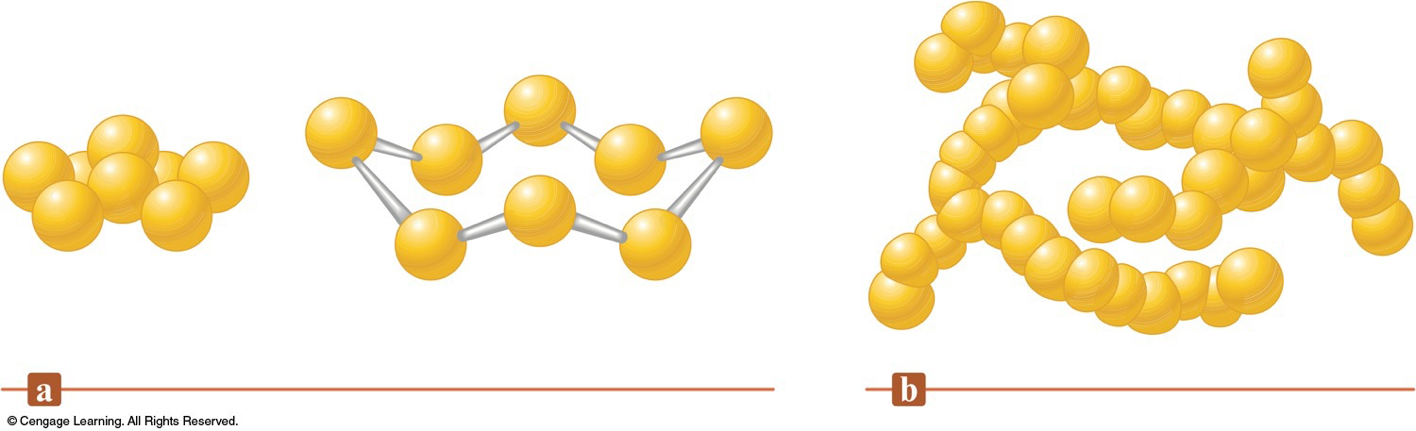
Sulfur
- Sulfur is found in nature both in large deposits of the free element and in ores such as galena, cinnabar, pyrite, gypsum, epsomite, and glauberite.
Frasch Process
Aggregates of Sulfur
Sulfur Oxide Reactions
$$ \begin{align} \chem{2SO_2(g)+O_2(g)} & \rightarrow \chem{2SO_3(g)} \\ \chem{SO_2(g)+H_2O(l)} & \rightarrow \chem{H_2SO_3(aq)} \\ \chem{SO_3(g)+H_2O(l)} & \rightarrow \chem{H_2SO_4(aq)} \end{align} $$
Halogens
- All nonmetals: \(\chem{F}\), \(\chem{Cl}\), \(\chem{Br}\), \(\chem{I}\), \(\chem{At}\)
- Because of their high reactivities, the halogens are not found as free elements in nature. They are found as halide ions (\(\chem{X^–}\)) in various minerals and in seawater.
Trends in Selected Physical Properties of the Group 7A Elements
| Element | Electronegativity | Radius of \(\chem{X^-}\) (pm) | \(\mathcal{E}^\circ\) (V) for \(\chem{X_2+2e^- \rightarrow 2X^-}\) | Bond Energy of \(\chem{X_2}\) (kJ/mol) |
|---|---|---|---|---|
| Fluorine | 4.0 | 136 | 2.87 | 154 |
| Chlorine | 3.2 | 181 | 1.36 | 239 |
| Bromine | 3.0 | 195 | 1.09 | 193 |
| Iodine | 2.7 | 216 | 0.54 | 149 |
| Astatine | 2.2 | - | - | - |
Some Physical Properties, Sources, and Methods of Preparation of the Group 7A Elements
| Element | Color and State | Percentage of Earth's Crust | Metling Point (\({}^\circ\mathrm{C}\)) | Boiling Point (\({}^\circ\mathrm{C}\)) | Source | Method of Preparation |
|---|---|---|---|---|---|---|
| Fluorine | Pale yellow gas | 0.07 | -220 | -188 | Fluorospar (\(\chem{CaF_2}\)), cryolite (\(\chem{Na_3AlF_6}\)), fluorapatite (\(\chem{Ca_5(PO_4)_3F}\)) | Electrolysis of molten \(\chem{KHF_2}\) |
| Chlorine | Yellow-green gas | 0.14 | -101 | -34 | Rock salt (\(\chem{NaCl}\)), halite (\(\chem{NaCl}\)), sylvite (\(\chem{KCl}\)) | Electrolysis of aqueous \(\chem{NaCl}\) |
| Bromine | Red-brown liquid | \(2.5 \times 10^{-4}\) | -7.3 | 59 | Seawater, brine wells | Oxidation of \(\chem{Br^-}\) by \(\chem{Cl_2}\) |
| Iodine | Violet-black solid | \(3 \times 10^{-5}\) | 113 | 184 | Seaweed, brine wells | Oxidation of \(\chem{I^-}\) by electrolysis or \(\chem{MnO_2}\) |
Preparation of Hydrogen Halides
$$ \chem{H_2(g)+X_2(g)\rightarrow 2HX(g)} $$
- When dissolved in water, the hydrogen halides behave as acids, and all except hydrogen fluoride are completely dissociated.
Halogen Oxyacids and Oxyanions
- All halogens except fluorine combine with various numbers of oxygen atoms to form a series of oxyacids.
- The strengths of these acids vary in direct proportion to the number of oxygen atoms attached to the halogen, with the acid strength increasing as more oxygens are added.
The Known Oxyacids of the Halogens
| Oxidation State of Halogen | Fluorine | Chlorine | Bromine | Iodine* | General Name of Acids | General Name of Salts |
|---|---|---|---|---|---|---|
| +1 | \(\chem{HOF}\)† | \(\chem{HOCl}\) | \(\chem{HOBr}\) | \(\chem{HOI}\) | Hypohalous acid | Hypohalites, \(\chem{MOX}\) |
| +3 | ‡ | \(\chem{HOClO}\) | ‡ | ‡ | Halous acid | Halites, \(\chem{MXO_2}\) |
| +5 | ‡ | \(\chem{HOClO_2}\) | \(\chem{HOBrO_2}\) | \(\chem{HOIO_2}\) | Halic acid | Halates, \(\chem{MXO_3}\) |
| +7 | ‡ | \(\chem{HOClO_3}\) | \(\chem{HOBrO_3}\) | \(\chem{HOIO_4}\) | Perhalic acid | Perhalates, \(\chem{MXO_4}\) |
* Iodine also forms \(\chem{H_4I_2O_9}\) (mesodiperiodic acid) and \(\chem{H_5IO_6}\) (paraperiodic acid).
† \(\chem{HOF}\) oxidation state is best represented as -1.
‡ Compound is unknown.
Noble Gases
- Filled s and p valence orbitals
- \(\chem{He}\) and \(\chem{Ne}\) form no compounds.
- \(\chem{Kr}\) and \(\chem{Xe}\) have been observed to form chemical compounds: $$ \begin{align} & \chem{Xe(g)+2F_2(g) \rightarrow XeF_4(s)}\;\;\;[\text{at 6 atm, }400^\circ\mathrm{C}] \\ & \chem{XeF_6(s)+3H_2O(l) \rightarrow XeO_3(aq)+6HF(aq)} \end{align} $$
Selected Properties of Group 8A Elements
| Element | Melting Point (\({}^\circ\mathrm{C}\)) | Boiling Point (\({}^\circ\mathrm{C}\)) | Atmoshperic Abundance (% by volume) | Example of Compounds |
|---|---|---|---|---|
| Helium | -270 | -269 | \(5 \times 10^{-4}\) | None |
| Neon | -249 | -246 | \(1 \times 10^{-3}\) | None |
| Argon | -189 | -186 | \(9 \times 10^{-1}\) | \(\chem{HArF}\) |
| Krypton | -157 | -153 | \(1 \times 10^{-4}\) | \(\chem{KrF_2}\) |
| Xenon | -112 | -107 | \(9 \times 10^{-6}\) | \(\chem{XeF_4}\), \(\chem{XeO_3}\), \(\chem{XeF_6}\) |
Concept Check
Which of the following groups is the most reactive? Group 1A, Group 5A, Group 6A, or Group 8A?
Group 1A Elements
Concept Check
White of the following groups does not contain at least one element that forms compounds with oxygen? Group 4A, Group 5A, Group 6A, Group 7A?
All of these groups contain at least one element that forms compounds with oxygen.
/