Chapter 12
Kinetics
Shaun Williams, PhD
Chemical Equilibrium
- The state where the concentrations of all reactants and products remain constant with time.
- On the molecular level, there is frantic activity. Equilibrium is not static, but is a highly dynamic situation.
- Equilibrium is:
- Macroscopically static
- Microscopically dynamic
The Changes with Time in the Rates of Forward and Reverse Reactions
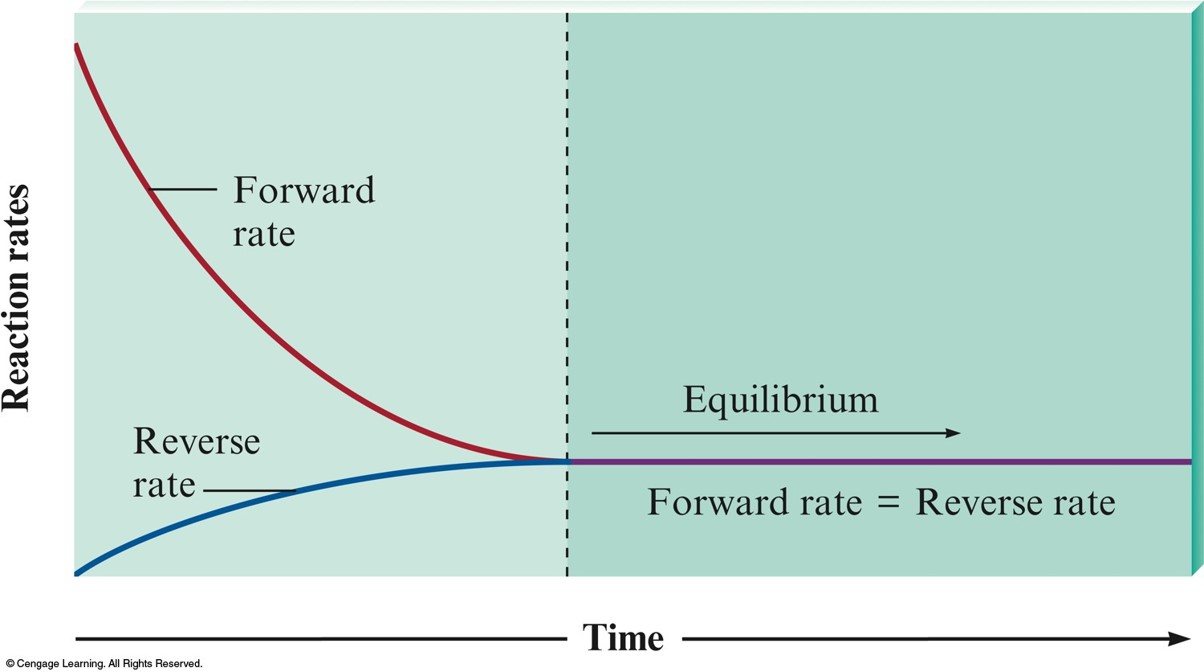
Consider the following reaction at equilibrium
$$ j\chem{A}+k\chem{B}\rightleftharpoons l\chem{C}+m\chem{D} $$ $$ K=\frac{\conc{C}^l\conc{D}^m}{\conc{A}^j\conc{B}^k} $$
- \(\chem{A,\,B,\,C,\,D}\) - chemical species
- Square brackets - concentrations of species at equilibrium
- \(j,\,k,\,l,\,m\) - coefficients in the balanced equation
- \(K\) - equilibrium constant (given without units)
Conclusions About the Equilibrium Expression
- Equilibrium expression for a reaction is the reciprocal of that for the reaction written in reverse.
- When the balanced equation for a reaction is multiplied by a factor of \(n\), the equilibrium expression for the new reaction is the original expression raised to the nth power; thus \(K_\mathrm{new}=\left(K_\mathrm{original}\right)^n\).
- \(K\) values are usually written without units.
- \(K\) always has the same value at a given temperature regardless of the amounts of reactants or products that are present initially.
- For a reaction, at a given temperature, there are many equilibrium positions but only one equilibrium constant, \(K\).
- Equilibrium position is a set of equilibrium concentrations.
Example
$$\chem{N_2(g)+3H_2(g)\rightleftharpoons 2NH_3(s)} $$ $$ \begin{align} K &= \frac{\conc{NH_3}^2}{\conc{N_2}\conc{H_2}^3} \\ K_\mathrm{P} &= \frac{\left(P_\chem{NH_3}\right)^2}{\left(P_\chem{N_2}\right)\left(P_\chem{H_2}\right)^3} \end{align} $$
The Relationship Between \(K\) and \(K_\mathrm{P}\)
$$ K_\mathrm{P}=K\left(RT\right)^{\Delta n} $$
- \(\Delta n\) = sum of the coefficients of the gaseous products minus the sum of the coefficients of the gaseous reactants.
- \(R = 0.08206\,\bfrac{\mathrm{L\cdot atm}}{\mathrm{mol\cdot K}}\)
- \(T\) = temperature (in Kelvin)
Types of Equilibria
- Homogeneous equilibria - involve the same phase: $$ \chem{N_2(g)+3H_2(g) \rightleftharpoons 2NH_3(g)} $$ $$ \chem{HCN(aq) \rightleftharpoons H^+(aq) + CN^-(aq)} $$
- Heterogeneous equilibria – involve more than one phase: $$ \chem{2KClO_3(s) \rightleftharpoons 2KCl(s) + 3O_2(g)} $$ $$ \chem{2H_2O(l) \rightleftharpoons 2H_2(g) + O_2(g)} $$
Heterogeneous Equilibria
- The position of a heterogeneous equilibrium does not depend on the amounts of pure solids or liquids present.
- The concentrations of pure liquids and solids are constant.
The Extent of a Reaction
- A value of \(K\) much larger than 1 means that at equilibrium the reaction system will consist of mostly products – the equilibrium lies to the right.
- Reaction goes essentially to completion.
- A very small value of \(K\) means that the system at equilibrium will consist of mostly reactants – the equilibrium position is far to the left.
- Reaction does not occur to any significant extent.
Reaction Quotient, \(Q\)
- Used when all of the initial concentrations are nonzero
- Apply the law of mass action using initial concentrations instead of equilibrium concentrations.
- \(Q = K\): The system is at equilibrium. No shift will occur.
- \(Q > K\): The system shifts to the left.
- Consuming products and forming reactants, until equilibrium is achieved.
- \(Q < K\): The system shifts to the right.
- Consuming reactants and forming products, to attain equilibrium.
Set Up ICE Table
$$ \chem{Fe^{3+}(aq)+SCN^-(aq) \rightleftharpoons FeSCN^{2+}(aq)} $$ $$ \begin{align} & \chem{Fe^{3+}(aq)} & + & \chem{SCN^-(aq)} & \rightleftharpoons & \chem{FeSCN^{2+}(aq)} \\ \mathrm{Initial}\;\; & 6.00 & & 10.00 & & 0.00 \\ \mathrm{Change}\;\; & -4.00 & & -4.00 & & +4.00 \\ \mathrm{Equilibrium}\;\; & 2.00 & & 6.00 & & 4.00 \end{align} $$ $$ K=\frac{\conc{FeSCN^{2+}}}{\conc{Fe^{3+}}\conc{SCN^-}} = \frac{(4.00\,\mathrm{M})}{(2.00\,\mathrm{M})(6.00\,\mathrm{M})} = 0.333 $$
Solving Equilibrium Problems
- Write the balanced equation for the reaction.
- Write the equilibrium expression using the law of mass action.
- List the initial concentrations.
- Calculate \(Q\), and determine the direction of the shift to equilibrium.
- Define the change needed to reach equilibrium, and define the equilibrium concentrations by applying the change to the initial concentrations.
- Substitute the equilibrium concentrations into the equilibrium expression, and solve for the unknown.
- Check your calculated equilibrium concentrations by making sure they give the correct value of \(K\).
Le Châtelier's Principle
- If a change is imposed on a system at equilibrium, the position of the equilibrium will shift in a direction that tends to reduce that change.
- Effects of Changes on the System
- Concentration: The system will shift away from the added component. If a component is removed, the opposite effect occurs.
- Temperature: \(K\) will change depending upon the temperature (endothermic – energy is a reactant; exothermic – energy is a product).
- Pressure:
- The system will shift away from the added gaseous component. If a component is removed, the opposite effect occurs.
- Addition of inert gas does not affect the equilibrium position.
- Decreasing the volume shifts the equilibrium toward the side with fewer moles of gas.
/