Chapter 6
Quantities in Chemical Reactions
Shaun Williams, PhD
The Meaning of a Balanced Equation
What do the coefficients in a balanced chemical equation mean?
- Let's consider the combustion of propane:
\[ \chem{C_3H_8(g) + O_2(g) \rightarrow CO_2(g) + H_2O(g)} \]
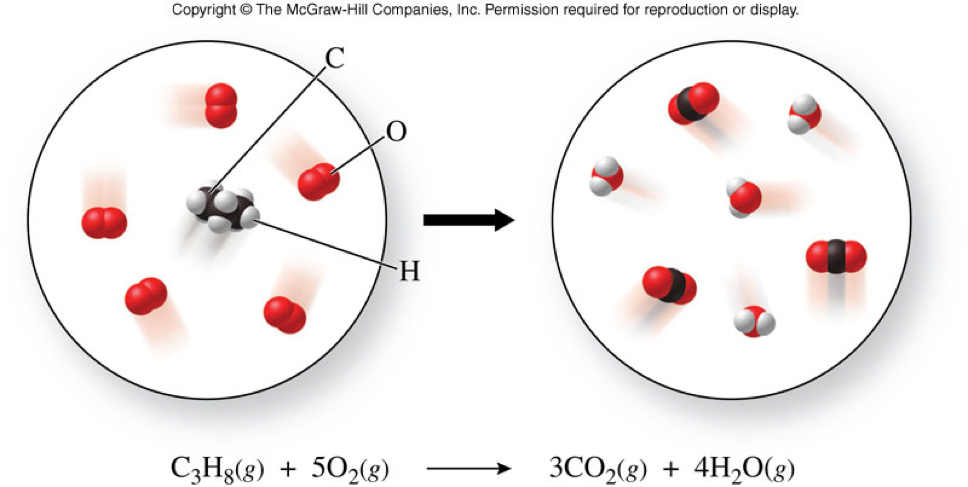
The Meaning of the Coefficients
| \( \chem{C_3H_8(g)} \) | \( + \) | \( \chem{5O_2(g)}\) | \( \rightarrow \) | \( \chem{3CO_2(g)} \) | \( + \) | \( \chem{4H_2O(g)} \) |
|---|---|---|---|---|---|---|
| 1 molecule | 5 molecules | 3 molecules | 4 molecules | |||
| 2 molecules | 10 molecules | 6 molecules | 8 molecules | |||
| 100 molecules | 500 molecules | 300 molecules | 400 molecules | |||
| 1 mole | 5 moles | 3 moles | 4 moles |
Mole-to-Mole Conversions
- In mole-mole conversions, we relate the moles of a reactant or product to other reactants or products using a mole ratio.
- Mole ratios:
- are obtained from the coefficients in the balanced chemical equation.
- help us determine the moles of one substance in a reaction when the number of moles of another substance in the same reaction is known.
- are used as conversion factors in dimensional analysis problems.
Mass-to-Mass Conversions

- Typically, chemical measuring devices do NOT measure in moles.
- We are often given grams as our beginning unit in problems that use a mole ratio.
- Therefore, we need to convert from grams to moles 1st.
- The conversion factor we need to convert from grams to moles (or vice versa) is the molar mass (MM).
The Law of Conservation of Mass
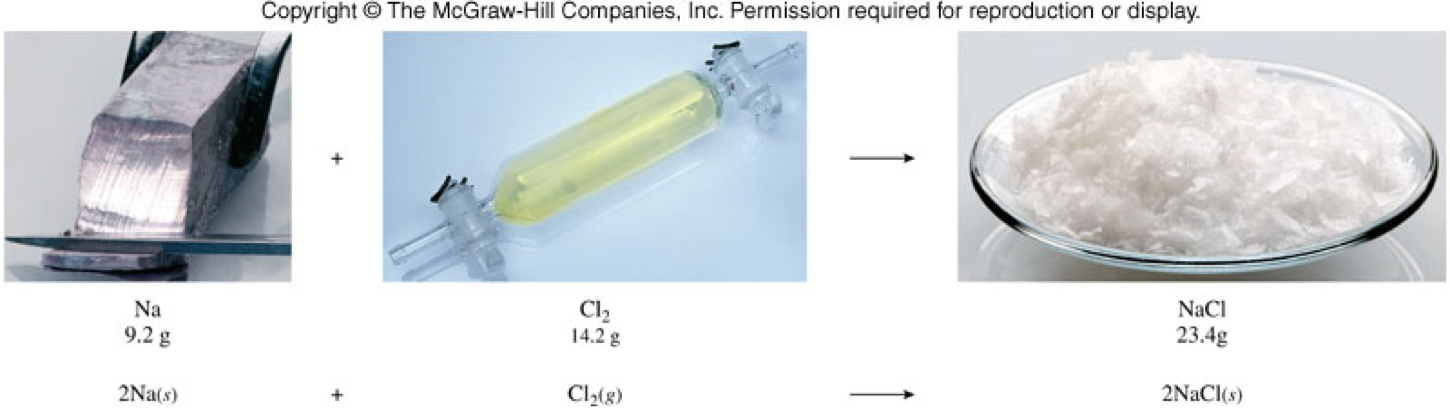
- The Law of Conservation of Mass states that the masses of the reactants must equal the masses of the products. \[ \text{Mass Reactants} = \text{Mass Products} \]
Limiting Reactants
What are limiting reactants?
- Take the equation: \[ \chem{2 Na(s) + Cl_2(g) \rightarrow 2 NaCl(s)} \]
- When reactants are not mixed in relative amounts as described by the balanced chemical equation, one reactant does not react completely.
- In this case, the two reactants are known as:
- Limiting reactant
- Reacts completely
- Limits the amount of the other reactant that can react
- Limits the amount of product that can be made
- Excess reactant
- DOES NOT react completely
- Limiting reactant
- In this case, the two reactants are known as:
Steps for Determining the Limiting Reactant
- Calculate the amount of one reactant (B) needed to react with the other reactant (A).
- Compare the calculated amount of B (amount needed) to the actual amount of B that is given.
- If calculated B = actual B, there is no limiting reactant. Both A and B will react completely.
- If calculated B > actual B, B is the limiting reactant. Only B will react completely.
- If calculated B < actual B, A is the limiting reactant. Only A will react completely.
Percent Yield
What is a percent yield?
- Percent yield
- Describes how much of a product is actually formed in comparison to how much should have been formed
- Theoretical yield
- The maximum amount of product that can be obtained from given amounts of reactants
- Actual yield
- The amount of product we measure in the laboratory
- Usually less than the theoretical yield
\[ \text{% yield} = \frac{\text{actual yield}}{\text{theoretical yield}} \times 100\% \]

Energy Changes
The Law of Conservation of Energy
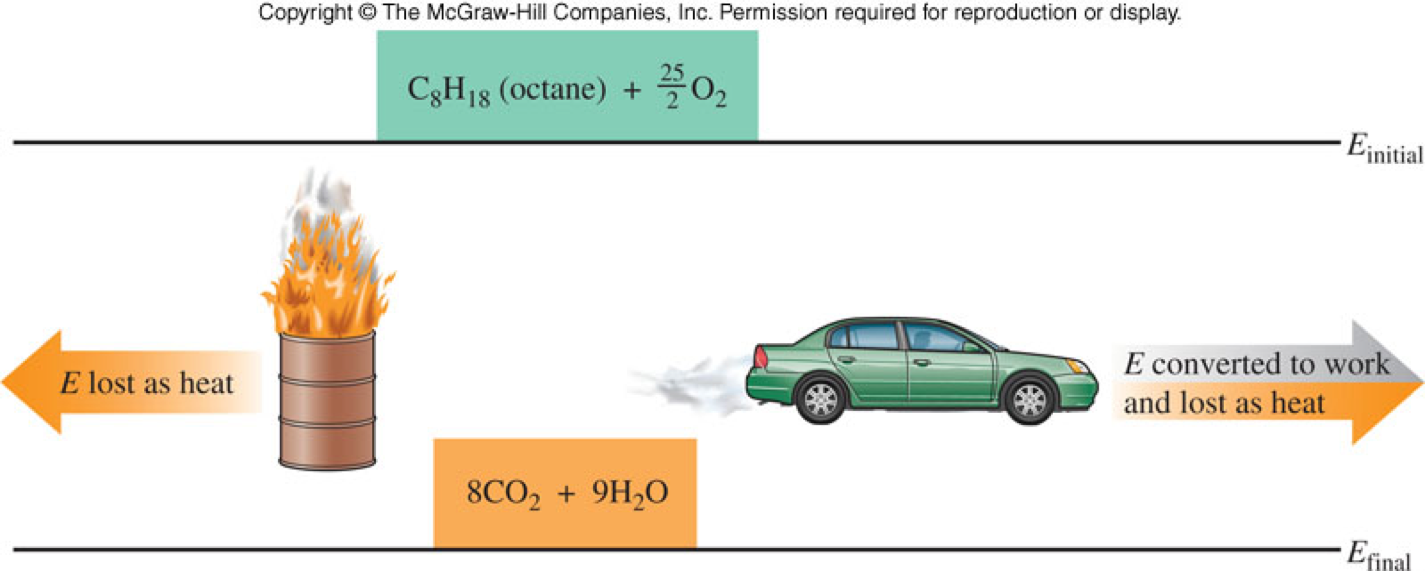
- Energy can be converted or transferred, but it cannot be created or destroyed.
- Heat is energy that is transferred between two objects because of a difference in their temperatures.
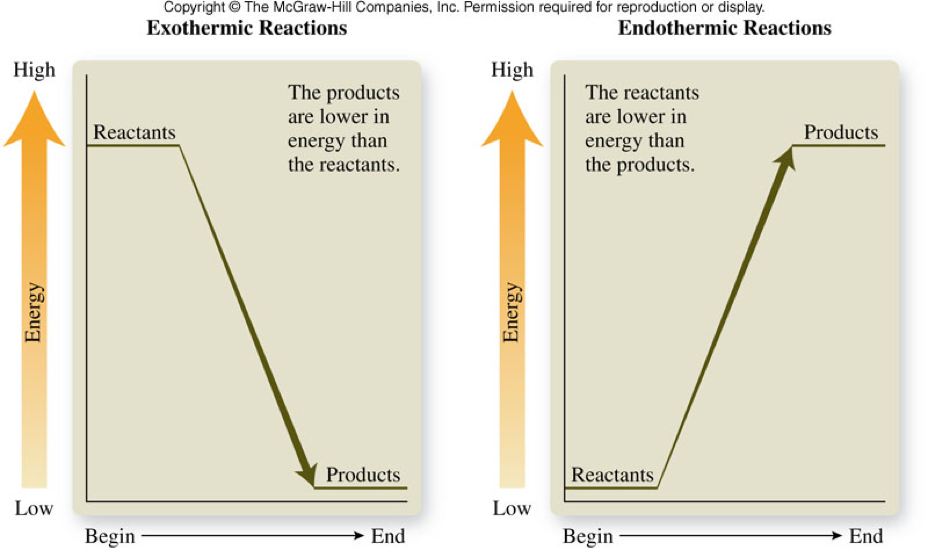
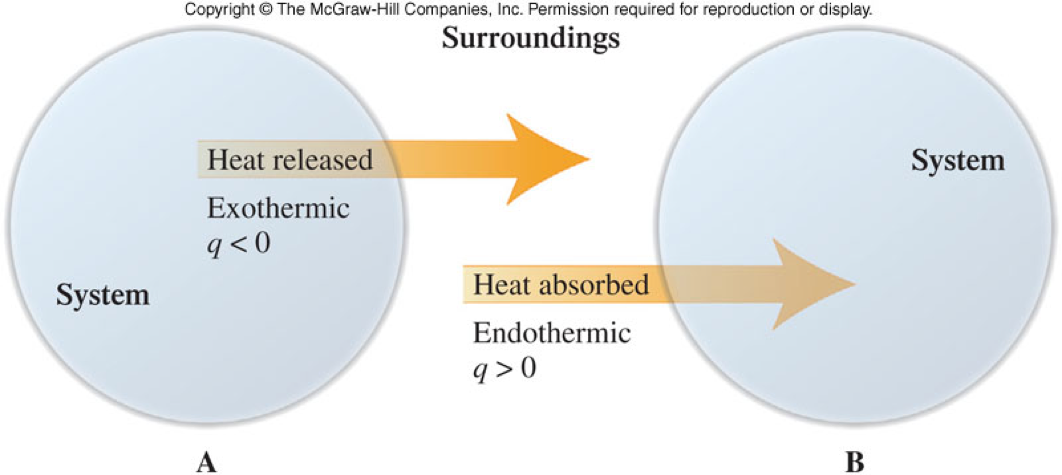
Exothermic and Endothermic Reactions
- Exothermic reaction
- A reaction that releases energy into the surroundings
- Endothermic reaction
- A reaction that absorbs energy from its surroundings
Specific Heat
- The amount of heat that must be added to \( \chem{1\, g} \) of a substance to raise its temperature by \( 1^\circ C\).
- Units are Joules per gram per degree Celsius [\( \bfrac{\text{J}}{\chem{g\,{}^\circ C}} \)]
- Is specific to the substance. See Table 6.2 for some common specific heats. \[ q = mC \Delta T \] where \(q\) is heat, \(m\) is mass, \(C\) is specific heat, and \(\Delta T\) is the change in temperature
Energy of the System and the Surroundings
\[ \chem{q_{system} + q_{surroundings} =0} \]
- A system can be an object such as a piece of pipe, or a process, such as a physical or chemical change.
- The surroundings are everything around the system.
Heat Changes in Chemical Reactions
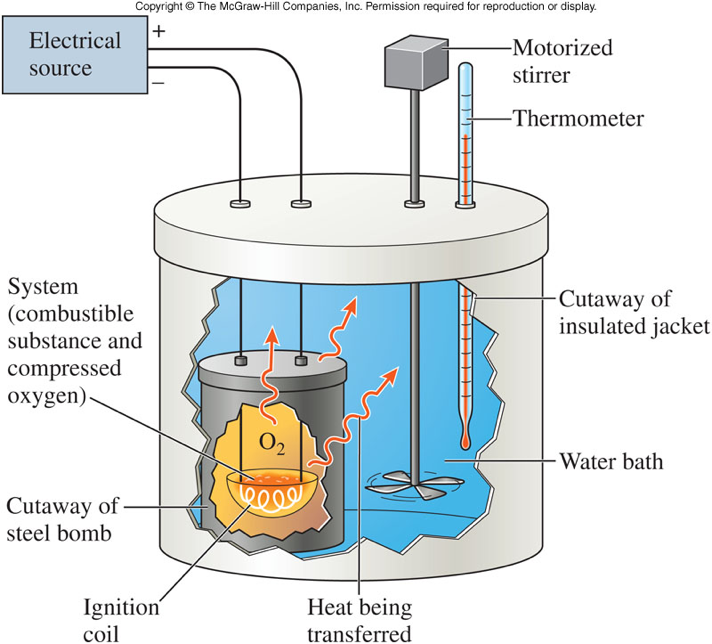
How we measure heat
- A bomb calorimeter is used to measure the heat transfer in a chemical reaction.
- Therefore, \[ \chem{q_{reaction} + q_{water} = 0} \] \[ \chem{q_{reaction} + q_{calorimeter} = 0} \]
/