Chapter 5
Chemical Reactions and Equations
Shaun Williams, PhD
Chemical Reaction
What is a chemical reaction?
- Chemical reaction
- The conversion of one substance or set of substances into another
- Reactant
- Any substance that is started with is called a reactant
- Product
- New substances that form during the course of the reaction
- Products differ from reactants only in the arrangement of their component atoms

A Microscopic View of a Chemical Reaction
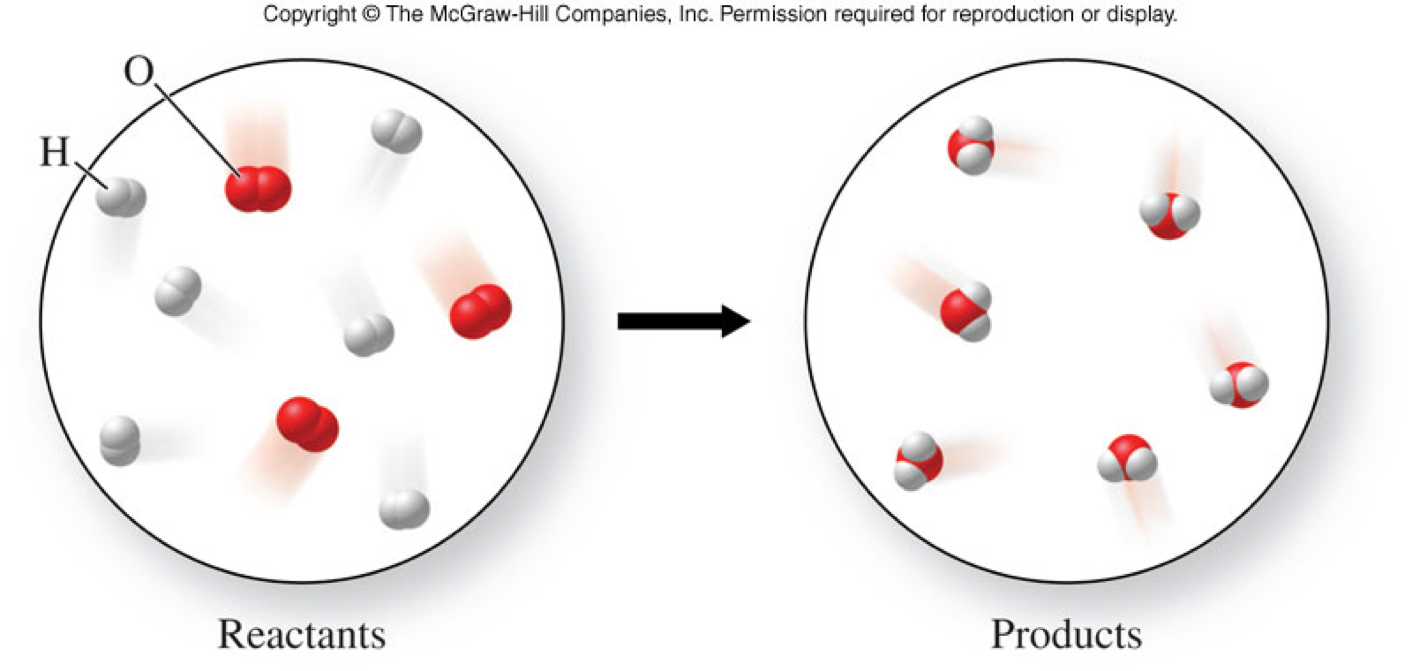
How Do We Know a Chemical Reaction Occurs?
What makes it a chemical reaction?
Do any of the pictures below show a chemical reaction? How can you tell?

Evidence of a Chemical Reaction
- The most common evidence of a chemical reaction is:
- Change in color
- Production of light
- Formation of a solid (such as a precipitate in solution, or smoke in air, or a metal coating)
- Formation of a gas (bubbles in solution or fumes in the gaseous state)
- Absorption or release of heat (sometimes appearing as a flame)
Writing Chemical Equations
What are chemical equations?
- Chemical equation
- A symbolic representation of a chemical reaction
- Balanced equation
- The number of atoms of each element is the same in the products as in the reactants
- Conservation of mass is always maintained
A General Approach to Balancing Equations
- Identify the reactants and products and write their correct formulas.
- Write a skeletal equation including physical states.
- Change coefficients one at a time until the atoms of each element are balanced. (Start with the elements that occur least often in the equation)
- Make a final check by counting the atoms of each element on both sides of the equation.
Writing Chemical Equations - Example 1
\[ \text{Aluminum + iron(III) oxide} \rightarrow \text{aluminum oxide + iron} \] \[ \chem{Al(s) + Fe_2O_3(s) \rightarrow Al_2O_3(s) + Fe(s)} \]
| # of atoms (reactants) | # of atoms (products) |
|---|---|
| 1 Al | 2 Al |
| 2 Fe | 1 Fe |
| 3 O | 3 O |
Therefore, we need to balance the equation with coefficients: \[ \chem{2Al(s) + Fe_2O_3(s) \rightarrow Al_2O_3(s) + 2Fe(s)} \]
Writing Chemical Equations - Example 2
\[ \text{Methane + oxygen} \rightarrow \text{carbon dioxide + water} \] \[ \chem{CH_4(g) + O_2(g) \rightarrow CO_2(g) + H_2O(g)} \]
Currently, the number of atoms of each element is shown below. These numbers were obtained by multiplying the subscript to the right of the element's symbol by the stoichiometric coefficient.
| # of atoms (reactants) | # of atoms (products) |
|---|---|
| 1 C | 1 C |
| 4 H | 2 H |
| 2 O | 3 O |
Writing Chemical Equations - Example 2 Part 2
\[ \chem{CH_4(g) + O_2(g) \rightarrow CO_2(g) + H_2O(g)} \]
First, we look at the carbon atoms. Since the number of carbon atoms on the reactant side is already equal to the number of carbon atoms on the product side, we don't need to add coefficients.
Next, we look at the hydrogen atoms. Currently, there are four hydrogen atoms on the reactant side and 2 hydrogen atoms on the product side. Thus, we need to add a coefficient of 2 in front of water to make the hydrogen atoms equal.
\[ \chem{CH_4(g) + O_2(g) \rightarrow CO_2(g) + 2H_2O(g)} \]
Writing Chemical Equations - Example 2 Part 3
\[ \chem{CH_4(g) + O_2(g) \rightarrow CO_2(g) + 2H_2O(g)} \]
Finally, we look at the oxygen atoms. Currently, there are 2 oxygen atoms on the reactant side and 4 oxygen atoms (combined from carbon dioxide and water) on the product side. Thus, we add a coefficient of 2 in front of the oxygen gas.
\[ \chem{CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g)} \]
Now the reaction is balanced!
Predicting Classes of Reactions
Background
- If we depict elements and compounds with letters and spheres:
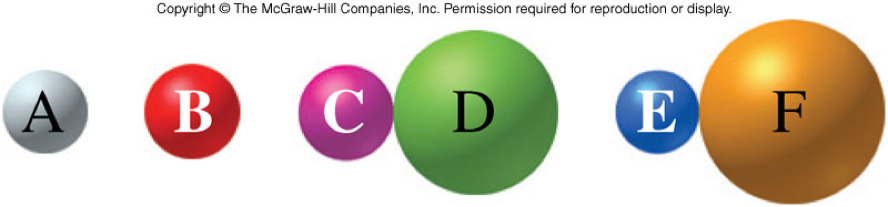 If A and B are elements (monatomic) and C, D, E, and F are possible atoms, monatomic ions, or polyatomic ions arranged to form molecules, then how many different types of chemical reactions can you identify by rearranging these atoms or groups of atoms? (NOTE: Do not react more than two elements or molecules)
If A and B are elements (monatomic) and C, D, E, and F are possible atoms, monatomic ions, or polyatomic ions arranged to form molecules, then how many different types of chemical reactions can you identify by rearranging these atoms or groups of atoms? (NOTE: Do not react more than two elements or molecules)
The Classes of Chemical Reactions
| Class | Reactants | Products | Example |
|---|---|---|---|
| Decomposition | 1 compound | 2 elements (or smaller compounds) | \( \chem{CD \rightarrow C+D} \) |
| Combination | 2 elements or compounds | 1 compounds | \( \chem{A + B \rightarrow AB} \) |
| Single-Replacement | 1 element + 1 compound | 1 elements + 1 compound | \( \chem{A + CD \rightarrow C+AD} \) |
| Double-Replacement | 2 compounds | 2 compounds | \( \chem{CD + EF \rightarrow CF+ED} \) |
Decomposition Reaction
- A compound breaks down into its component elements.
- Example: \[ \chem{2HgO(s) \xrightarrow{heat} 2Hg(l) + O_2(g)} \]

Decomposition Reaction - A Molecular View
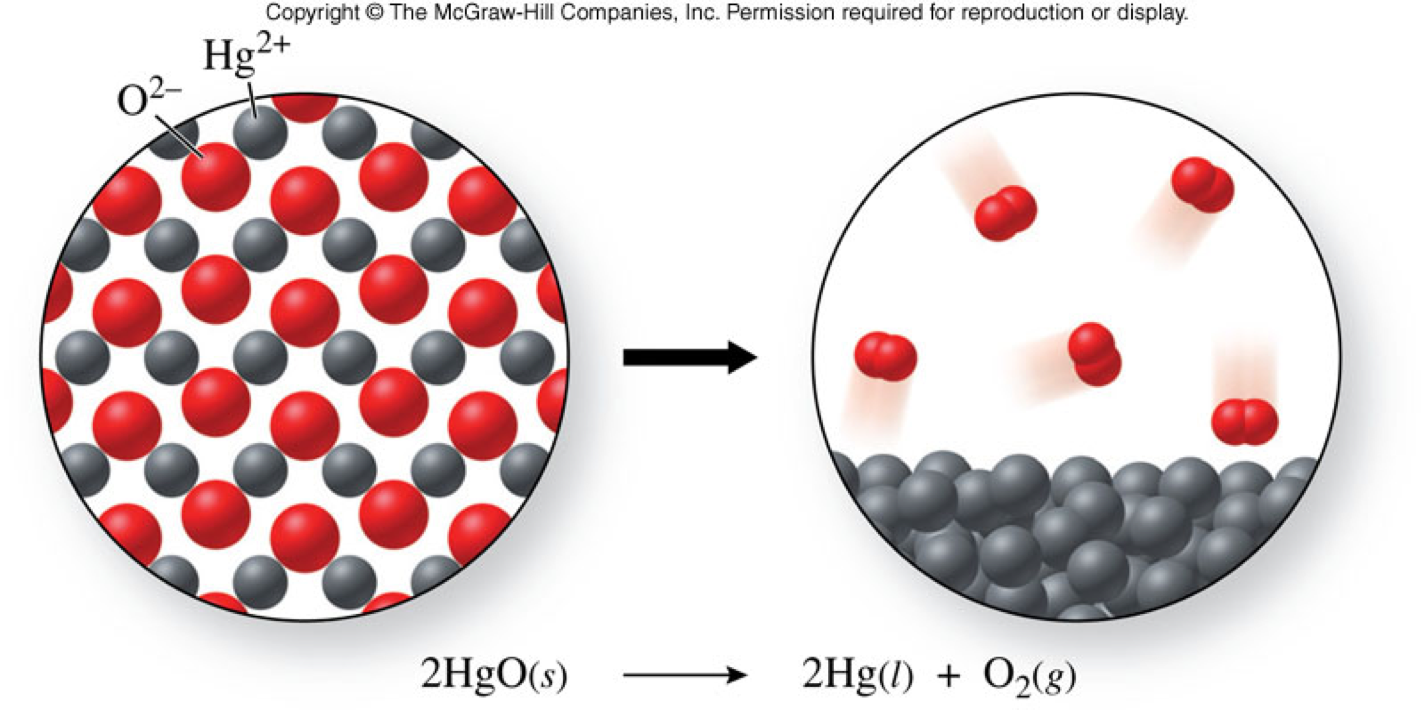
Decomposition Reactions That Occur When Compounds Are Heated
| Oxides and halides of the metals Au, Pt, and Hg decompose to the elements. \( \chem{2HgO(s) \rightarrow 2Hg(l) + O_2(g)} \) |
| Peroxides decompose to oxides and oxygen gas. \( \chem{2H_2O_2(aq) \rightarrow 2H_2O(l) + O_2(g)} \) |
| Metal carbonates, except those of group 1A metals, decompose to metal oxides and carbon dioxide gas. \( \chem{NiCO_3(s) \rightarrow NiO(s) + CO_2(g)} \) |
| Oxoacids decompose in a similar way to form nonmetal oxides and water. \( \chem{H_2CO_3(aq) \rightarrow H_2O(l) + CO_2(g)} \) |
| Ammonium compounds lose ammonia gas. \( \chem{\left( NH_4 \right)_2SO_4(s) \rightarrow NH_3(g) + H_2SO_4(l)} \) |
Combination Reactions
- Two elements, an element and a compound, or two compounds react to produce a single compound.
- Most metals react with most nonmetals to form ionic compounds.
- A nonmetal may react with a more reactive nonmetal to form a molecular compound.
- A compound and an element may combine to form another compound if one exists with a higher atom: atom ratio.
- Two compounds may react to form a new compound.
- Example: \( \chem{2Al(s) + 3Br_2(l) \rightarrow 2AlBr_3(s)} \)
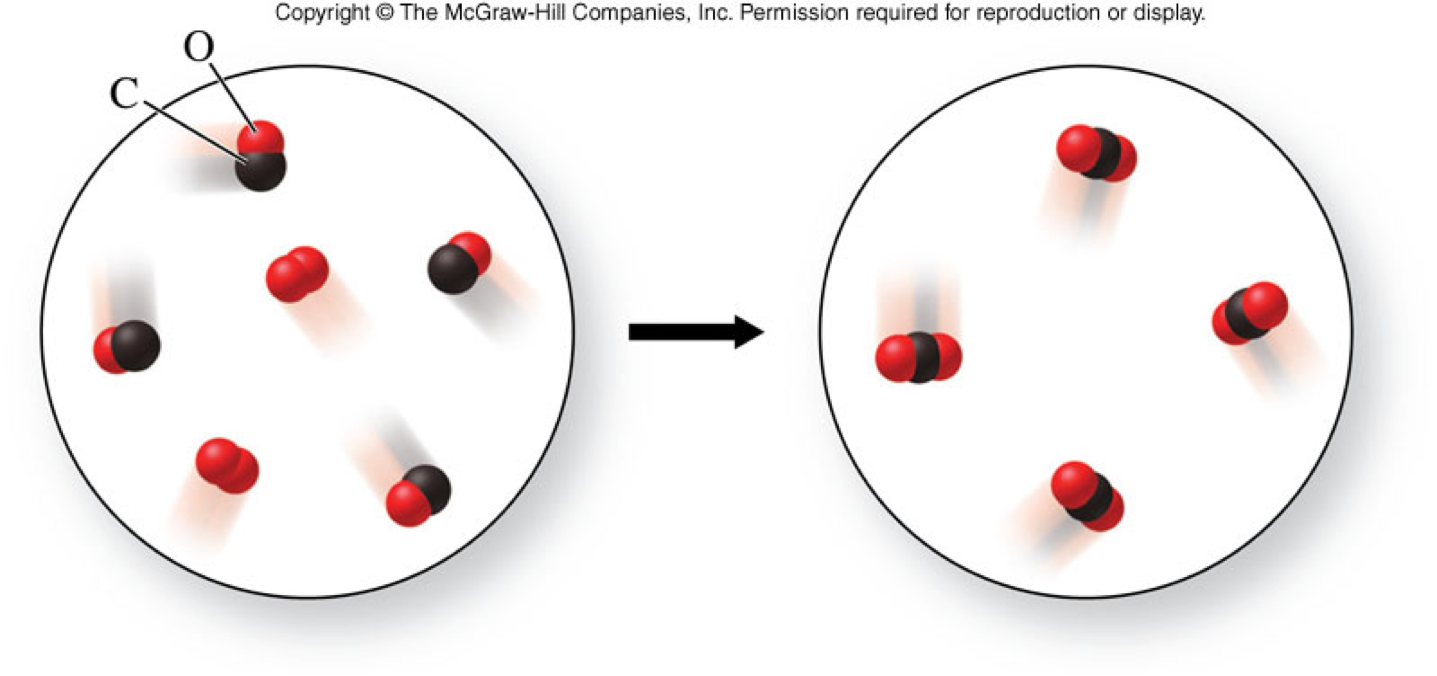
Combination Reaction - Example

Combination Reaction - A Molecular View
Single-Displacement Reaction
- A free element displaces another element from a compound to form another compound and a different free element.
- Example: \[ \chem{2Al(s) + Fe_2O_3(s) \rightarrow Al_2O_3(s) + 2 Fe(s)} \]

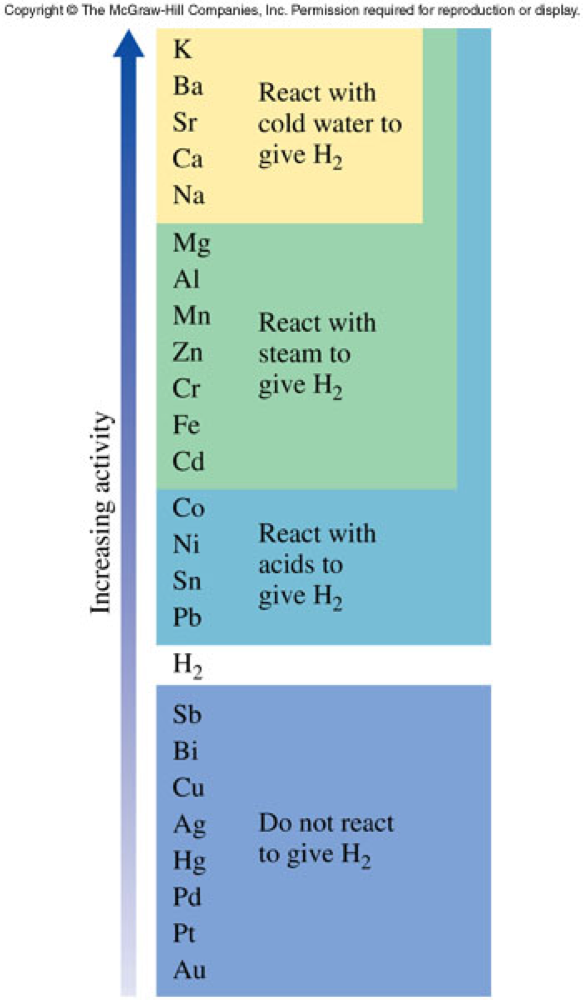
Activity Series
- This is a list of metals in order of their reactivity
- A more active element displaces a less active element from its compounds.
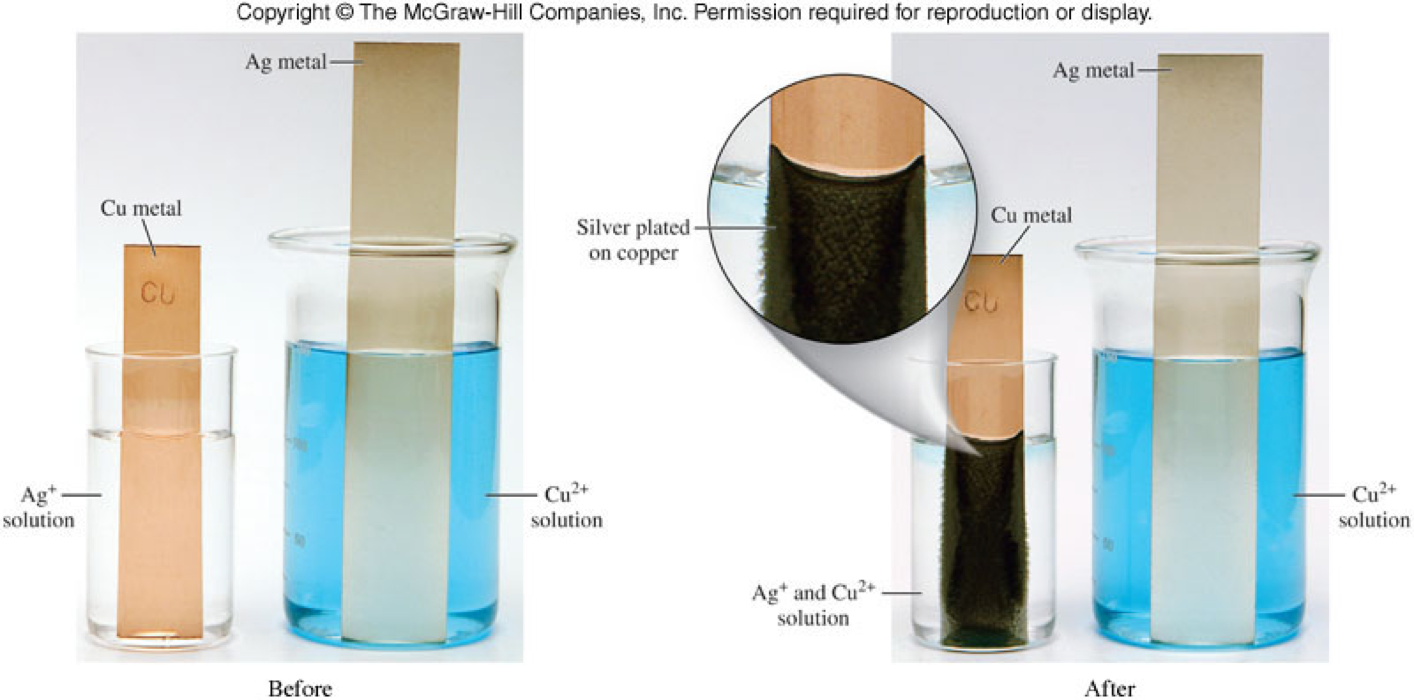
Single-Displacement Reactions: Copper and Silver Nitrate

Single-Displacement Reactions: Copper and Silver Nitrate - A Molecular View
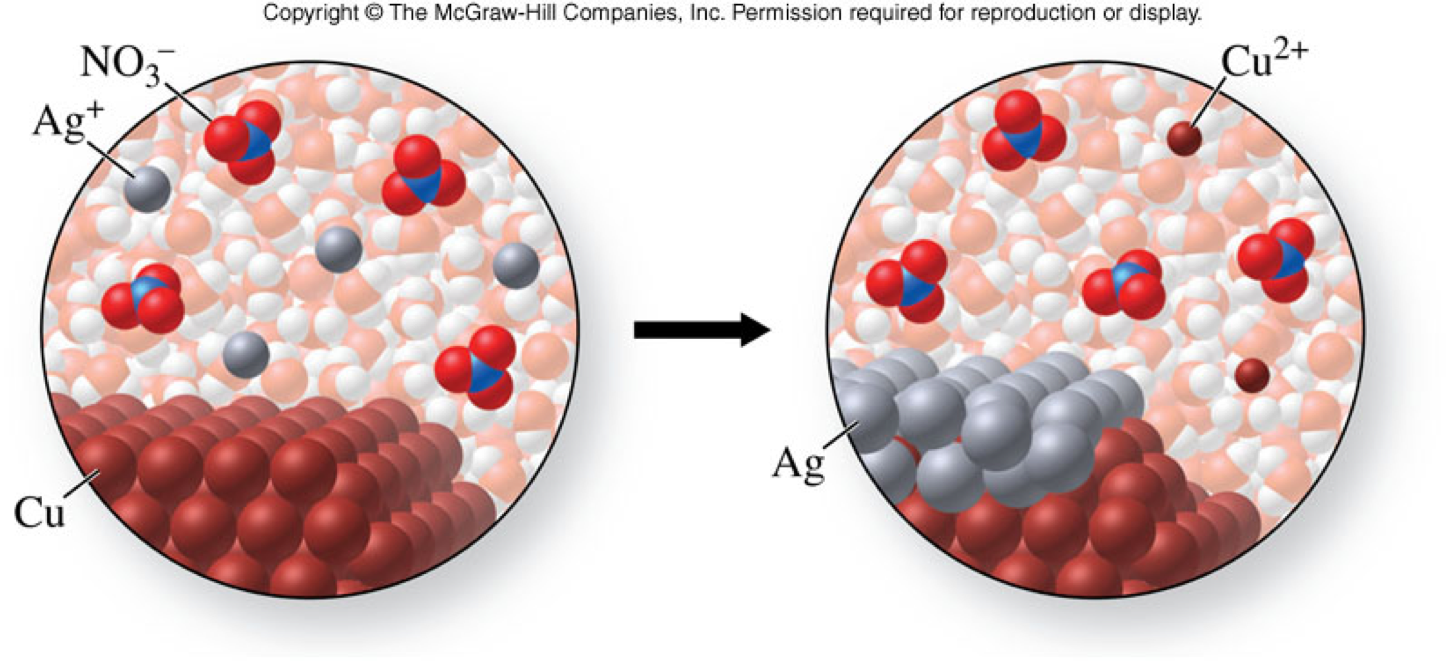
Single-Displacement Reactions: Copper and Silver Nitrate - Reversible?
Double-Displacement Reaction
- Two compounds exchange ions or elements to form new compounds.
- Precipitation reactions
- Gas-forming reactions
- Acid-Base Neutralizations
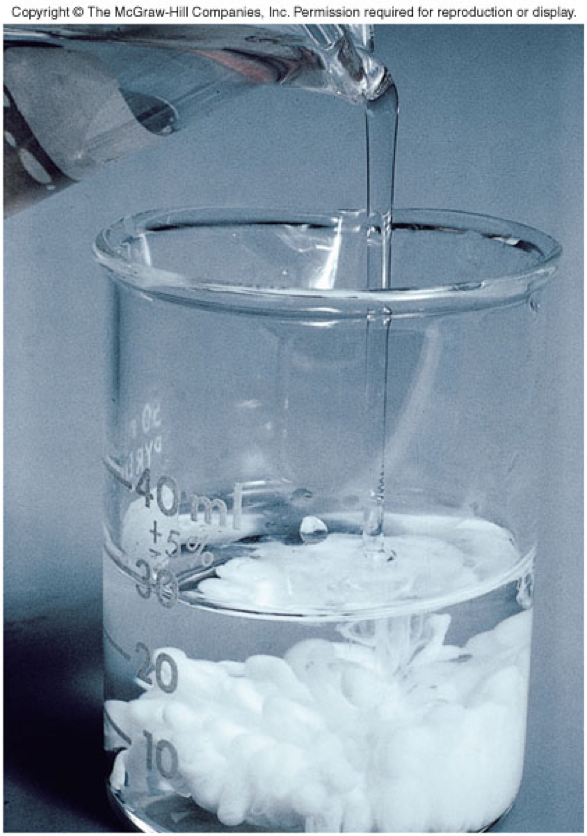
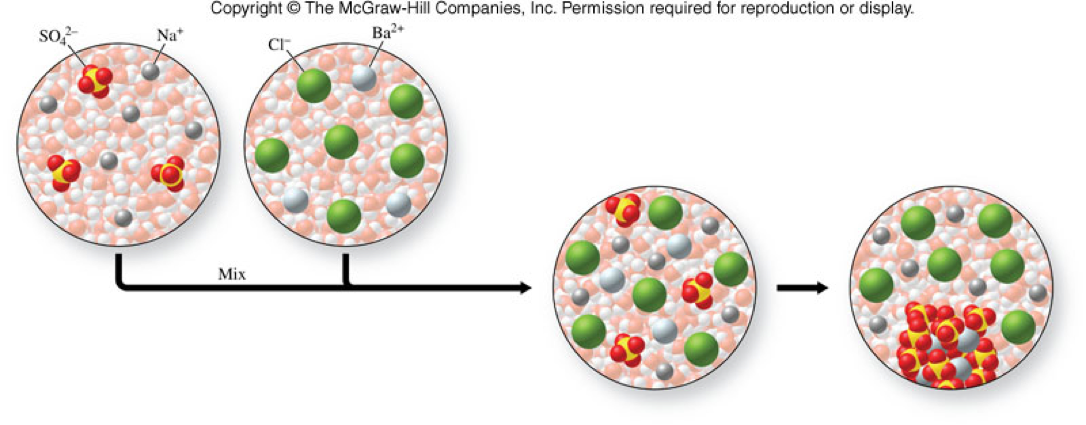
Precipitation Reactions
- When one product separates from the reaction solution, it is insoluble.
- An insoluble compound formed in a reaction is a precipitate.
- Example:
\[ \chem{BaCl_2(aq) + Na_2SO_4(aq) \rightarrow 2NaCl(aq) + BaSO_4(s)} \]
Solubility Rules
| Ions | Rule |
|---|---|
| \( \chem{Na^+ ,\, K^+ ,\, NH_4^+} \) (and other alkali metal ions) | Most compounds of alkali metal and ammonium ions are soluble. |
| \( \chem{NO_3^- ,\, C_2H_3O_2^-} \) | All nitrates and acetates are soluble. |
| \( \chem{SO_4^{2-}} \) | Most sulfates are soluble. Exceptions are \( \chem{BaSO_4 ,\, SrSO_4 ,\, PbSO_4 ,\, CaSO_4 ,\, Hg_2SO_4 ,\, Ag_2SO_4}\). |
| \( \chem{Cl^- ,\, Br^- ,\, I^-} \) | Most chlorides, bromides, and iodides are soluble. Exceptions are \( \chem{AgX ,\, Hg_2X_2 ,\, PbX_2 ,\, HgI_2} \). (\( \chem{X=Cl ,\, Br ,\, I}\)) |
| \( \chem{Ag^+} \) | Silver compounds, except \( \chem{AgNO_3 \, and \, AgClO_4} \) are insoluble. \(\chem{AgC_2H_3O_2}\) is slightly soluble. |
| \( \chem{O^{2-} ,\, OH^-} \) | Oxides and hydroxides are insoluble. Exceptions are alkali metal hydroxides, \(\chem{Ba(OH)_2 ,\, Sr(OH)_2 ,\, Ca(OH)_2}\) (somewhat soluble) |
| \( \chem{S^{2-}} \) | Sulfides are insoluble. Exceptions are compounds of \(\chem{Na^+ ,\, K^+ ,\, NH_4^+}\) and the alkaline earth metal ions. |
| \( \chem{CrO_4^{2-}} \) | Most chromates are insoluble. Exceptions are compounds are \(\chem{Na^+ ,\, K^+ ,\, NH_4^+ ,\, Mg^{2+} ,\, Ca^{2+} ,\, Al^{3+} ,\, Ni^{2+}}\). |
| \( \chem{CO_3^{2-} ,\, PO_4^{3-} ,\, SO_3^{2-} ,\, SiO_3^{2-}} \) | Most carbonates, phosphates, sulfites, and silicates are insoluble. Exceptions are compounds of \(\chem{Na^+ ,\, K^+ ,\, NH_4^+}\). |
Gas-Forming Reactions
- When one product separates from the reaction solution, it is insoluble.
- One product compound separates from the reaction mixture because it forms a gas.
- Example: \[ \chem{CaCO_3(s) + 2HCl(aq) \rightarrow CaCl_2(aq) + CO_2(g) + H_2O(l)} \]
Acid-Base Reactions
- A double-displacement reaction involving an acid and a base.
- An acid reacts with a base to form an ionic compound and water.
- Example: \[ \chem{HCl(aq) + NaOH(aq) \rightarrow NaCl(aq) + H_2O(l)} \]
Combustion Reactions Examples
- Any reaction involving oxygen as a reactant and that rapidly produces heat and flame

Combustion Reactions Molecular Examples
- Example: \[ \chem{CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g)} \]
Representing Reactions in Aqueous Solution
Aqueous Reactions

How can we better represent reactions in a solution?
- When compounds exist in solution, they exist as ions.
- Insoluble compounds do not exist as ions. \[ \chem{Pb(NO_3)_2(aq) + K_2CrO_4(aq) \rightarrow PbCrO_4(s) + 2 KNO_3(aq)} \]
- Ionic Equation
- Separate every compound with an (aq) by it into component ions.
- You cannot separate solids, liquids, or gases. \[ \chem{Pb^{2+} + 2 NO_3^- + 2 K^+ + CrO_4^{2-} \rightarrow PbCrO_4 + 2 K^+ + 2 NO_3^-} \]
- Spectator Ions: ions that exist on both sides of the arrow
- Net Ionic Equation
- The remaining equation without the spectator ions \[ \chem{Pb^{2+}(aq) + CrO_4^{2-}(aq) \rightarrow PbCrO_4(s)} \]
/