Chapter 3
Chemical Compounds
Shaun Williams, PhD
Ionic and Molecular Compounds
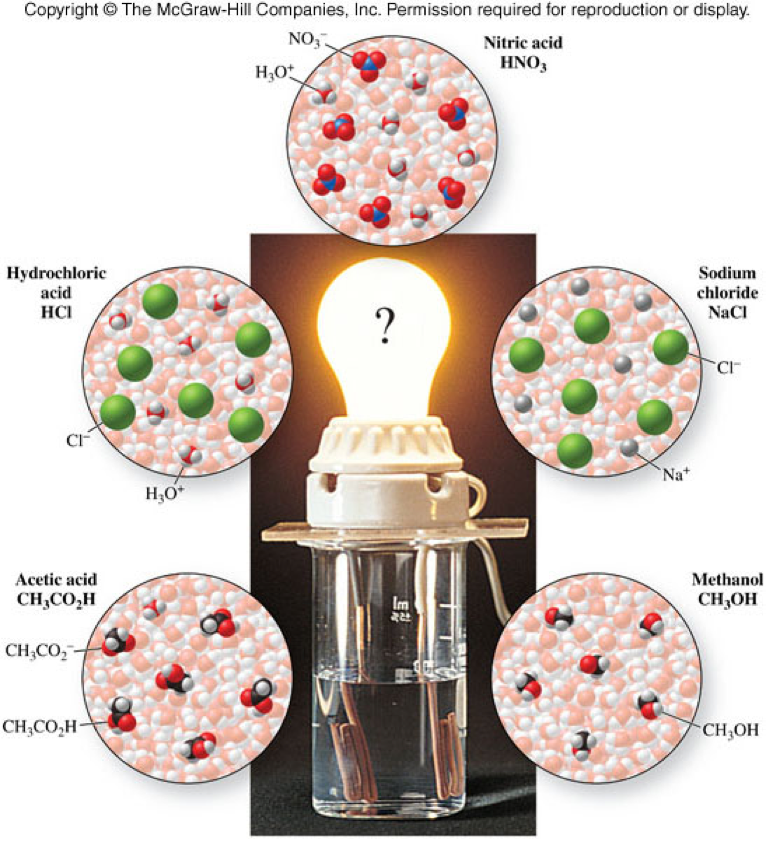
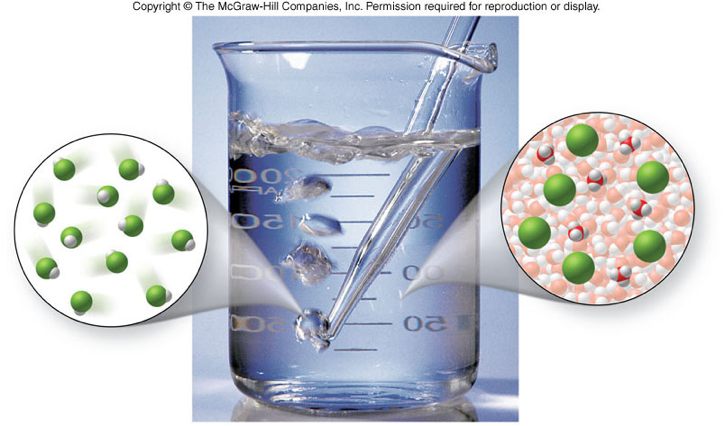
Electrolytes and Nonelectrolytes
- Electrolytes
- substances that release ions when dissolved in water
- This process is also called dissociation or ionization
- conduct electricity
- substances that release ions when dissolved in water
- Nonelectrolytes
- substances that do NOT dissociate in water
- do NOT conduct electricity
- Example: methanol
Strong and Weak Electrolytes
- Strong electrolytes
- dissociate completely into ions in water
- conduct electricity well
- Examples: NaCl and HCl
- Weak electrolytes
- do not dissociate completely into ions in water
- do not conduct electricity well
- Example: acetic acid
Molecular View of Electrolytes and Nonelectrolytes
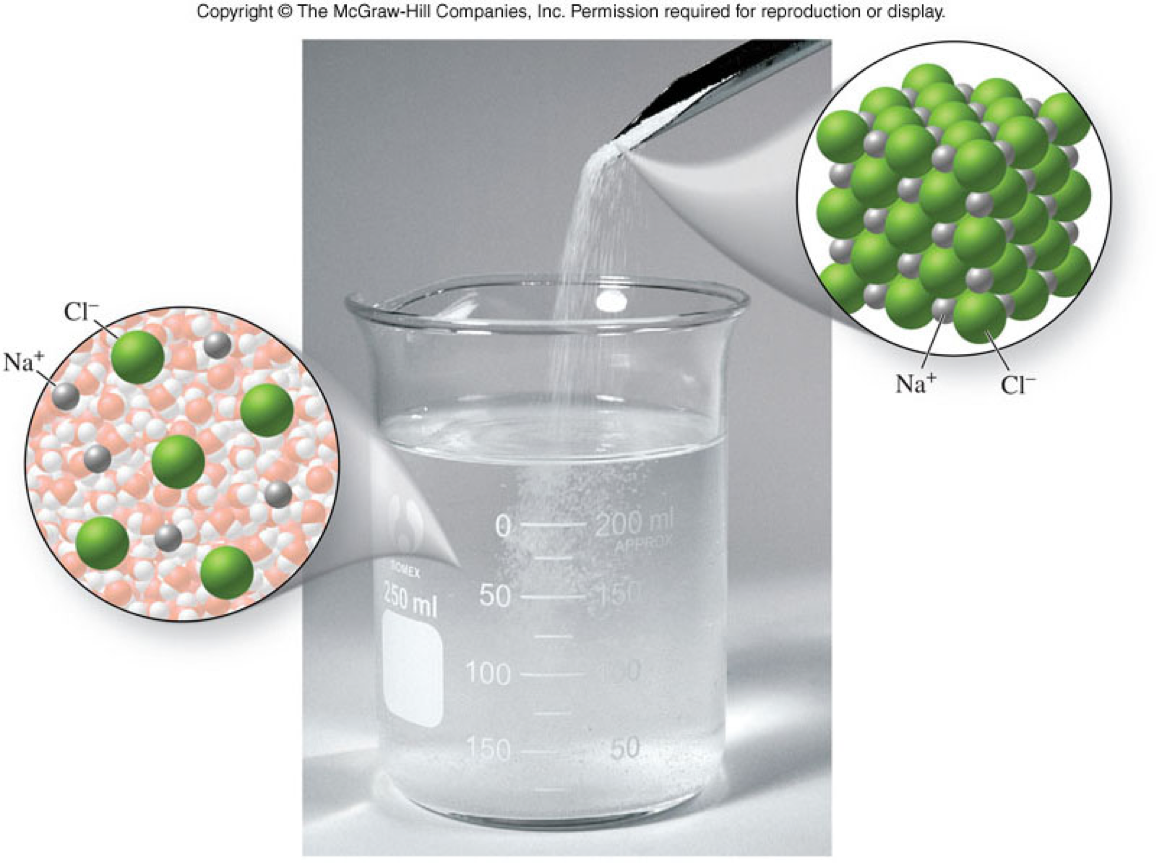
Ionic Compounds
- Are composed of a metal cation and a nonmetal anion
- Metals cations are
- positively charged ions
- Nonmetals anions are
- negatively charged ions
- Metals cations are
- Their cations and anions exist in proportions that give electrical neutrality
- These ions are arranged in a crystal lattice
- Also called salts
- Are one of the major categories of chemical compounds
Molecular View of Ionic Compounds
Molecular Compounds
- Are composed of 2 or more nonmetals
- Have no overall charge
- Are the other major category of chemical compounds
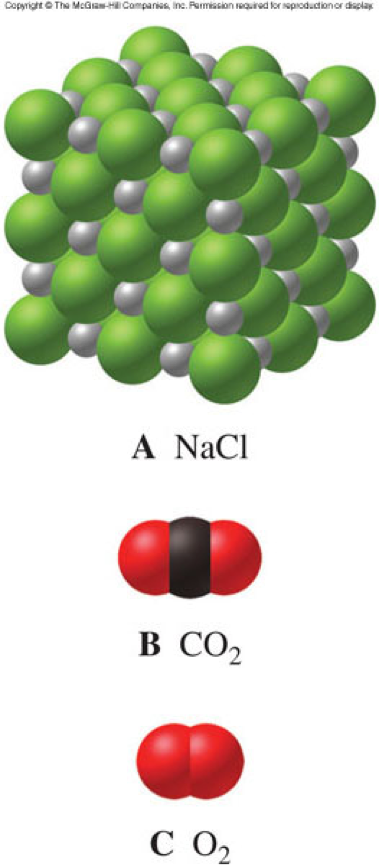
Ionic and Molecular Compound Properties
| Properties of Ionic and Molecular Compounds | |
|---|---|
| Ionic Compounds | Molecular Compounds |
| Crystalline solid | Gas, liquid, or solid |
| Hard, brittle solid | Soft solid |
| Very high melting point | Low melting point |
| Very high boiling point | Low boiling point |
| High density | Low density |
| Strong electrolyte in aqueous solution | Weak or nonelectrolyte in aqueous solution |
| Electrical conductivity is good when compound is molten | Electrical conductivity is poor in pure form |
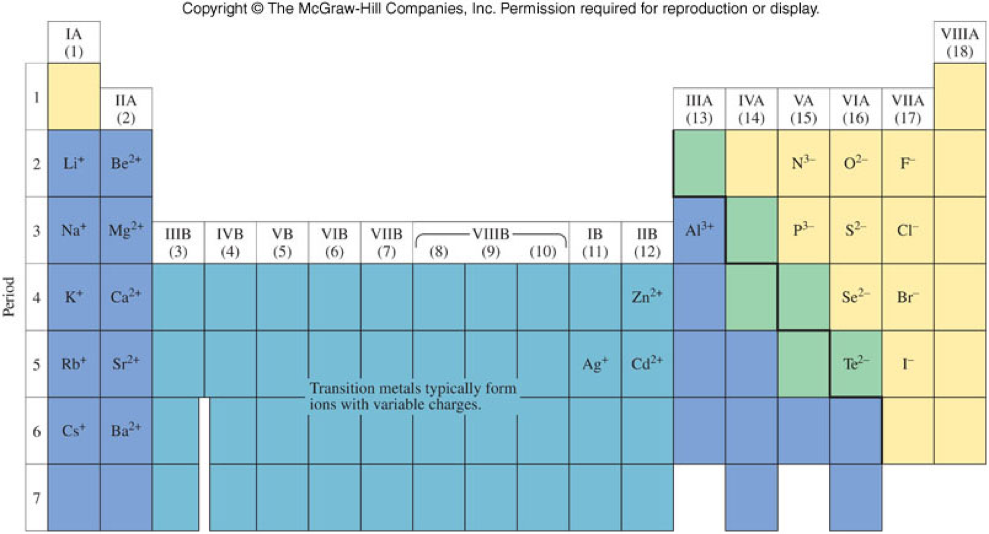
Monatomic and Polyatomic Ions
Monoatomic Ions
- Are ions of a single atom
- Many are shown below on Figure 3.12 and Table 3.2
- Most main-group elements tend to form their ion charge based on how far away their group is from the noble gases
- Transition metals tend to form multiple charges
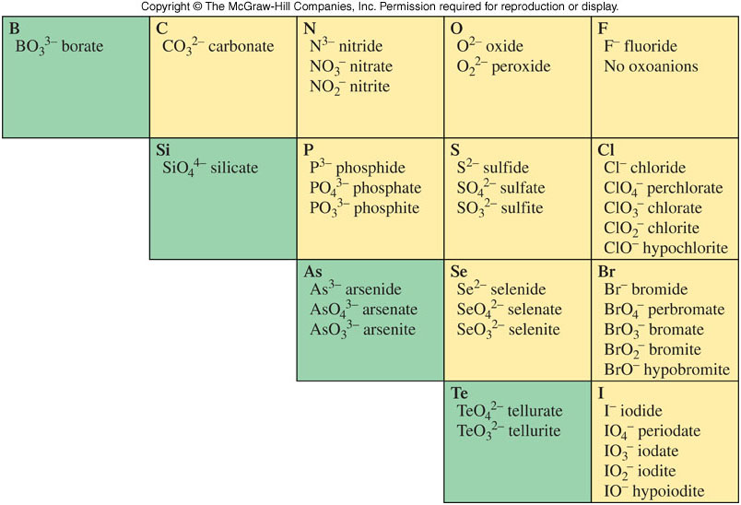
Polyatomic Ions
- An ion containing 2 or more atoms, usually of more than one element
- The most common are oxoanions, anions that contain oxygen attached to another element.
- Many are shown below on Figure 3.17 and Table 3.4
- One polyatomic cation – \( \chem{NH_4^+} \)
Important Polyatomic Anions
| Ions with a \(1-\) charge | Ions with a \(2-\) charge | ||
|---|---|---|---|
| Nitrate | \(\chem{NO_3^-}\) | Chromate | \(\chem{CrO_4^{2-}}\) |
| Nitrite | \(\chem{NO_2^-}\) | Dichromate | \(\chem{Cr_2O_7^{2-}}\) |
| Bicarbonate | \(\chem{HCO_3^-}\) | Sulfate | \(\chem{SO_4^{2-}}\) |
| Perchlorate | \(\chem{ClO_4^-}\) | Sulfite | \(\chem{SO_3^{2-}}\) |
| Chlorate | \(\chem{ClO_3^-}\) | Carbonate | \(\chem{CO_3^{2-}}\) |
| Chlorite | \(\chem{ClO_2^-}\) | Oxalate | \(\chem{C_2O_4^{2-}}\) |
| Hypochlorite | \(\chem{ClO^-}\) | Peroxide | \(\chem{O_2^{2-}}\) |
| Cyanide | \(\chem{CN^-}\) | Ions with a \(3-\) charge | |
| Hydroxide | \(\chem{OH^-}\) | Phosphate | \(\chem{PO_4^{3-}}\) |
| Acetate | \(\chem{C_2H_3O_2^-}\) | Phosphite | \(\chem{PO_3^{3-}}\) |
| Permanganate | \(\chem{MnO_4^-}\) | Borate | \(\chem{BO_3^{2-}}\) |
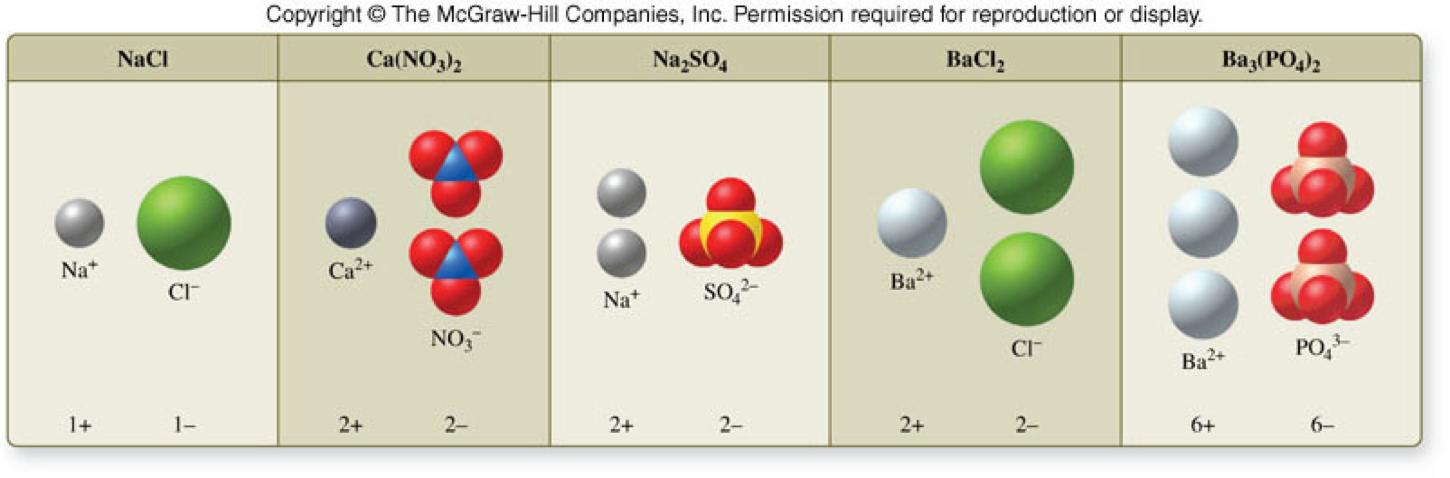
Formulas for Ionic Compounds
Writing Formulas for Ionic Compounds
- When writing a formula for an ionic compound, the sum of the positive charges must equal the sum of the negative charges. \[ \text{Total positive charge} + \text{total negative charge} = \text{zero net charge} \]
- The crystal structures or lattices are composed of repeating units called formula units.
Naming Ionic Compounds
How to name ionic compounds
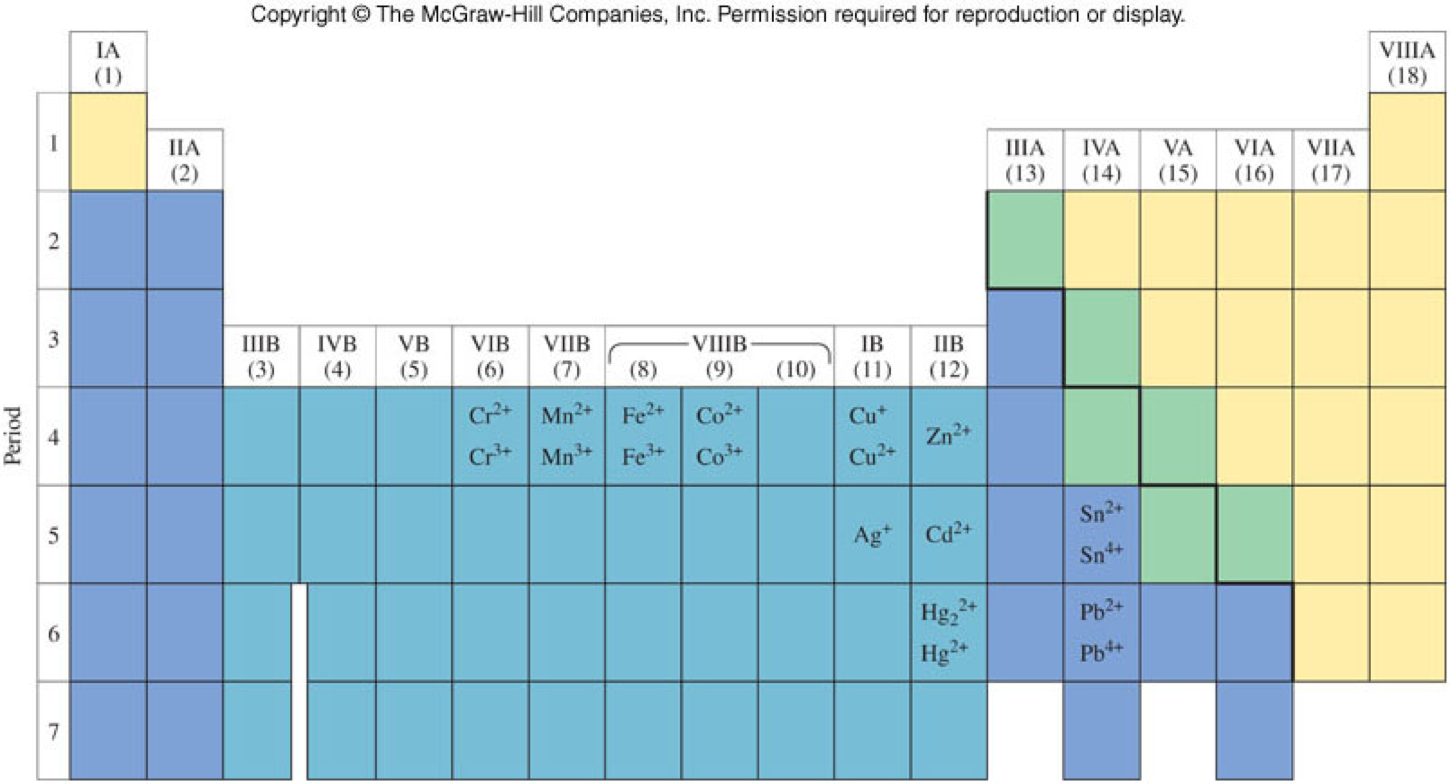
- Naming metals
- Monatomic cations are named according to the periodic table
- Transition metals (and Sn and Pb) require a Roman numeral in parenthesis to designate their charge (using the Stock System)
- Monatomic cations are named according to the periodic table
Summary of Main Group Metals
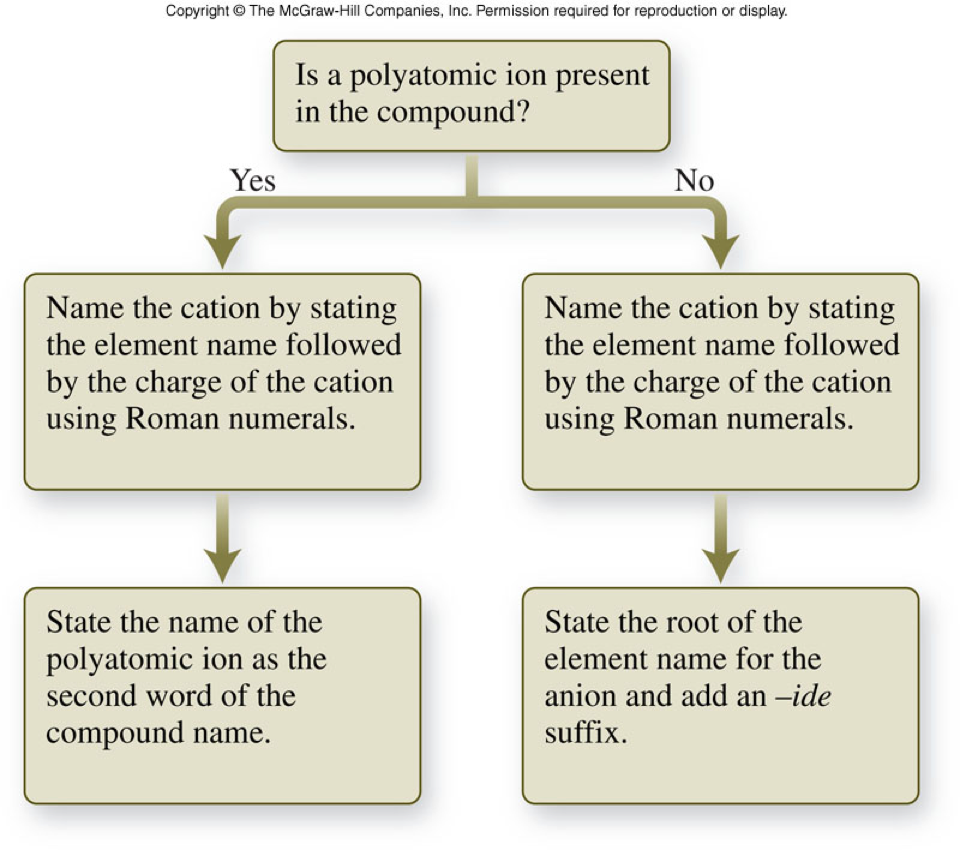
How to finish naming ionic compounds
- Naming nonmetals
- Monatomic anions
- Name according to the periodic table
- Drop the ending
- Add an –ide
- Polyatomic anions
- Name according to the Polyatomic Ion Chart (Table 3.4)
- Monatomic anions
Summary of Naming with Transition Metals
Naming and Writing Formulas for Molecular Compounds
Naming Molecular Compounds
- We will name binary molecular compounds
- Binary Compounds
- A compound containing atoms or ions of only two elements
- Binary Compounds
- To name molecular compounds, we:
- Name the leftmost element as we would a main-group metal – according to the periodic table.
- Name the rightmost element as we would a monatomic anion – drop the ending of the name (from the periodic table) and add an “-ide”.
- Use Greek prefixes to denote the number of atoms of each element.
Common Greek Prefixes
| Prefix | Name | Prefix | Name |
|---|---|---|---|
| mono- | 1 | hexa- | 6 |
| di- | 2 | hepta- | 7 |
| tri- | 3 | octa- | 8 |
| tetra- | 4 | nona- | 9 |
| penta- | 5 | deca- | 10 |
Summary of Naming Molecular Compounds

Acids and Bases
What are acids and bases?
- Acids
- Are substances that when dissolved in water provide hydrogen ions (\(\chem{H^+}\))
- An example of the dissociation (or ionization) of an acid: \[ \chem{HCl(aq) \xrightarrow{H_2O} H^+ (aq) + Cl^- (aq)} \]
- Bases
- Are substances that react with acids in aqueous solution to form water
- An example of the ionization of a base: \[ \chem{NaOH(s) \xrightarrow{H_2O} Na^+(aq) + OH^-(aq)} \]
Names of Some Common Acids
| Formula | Name | Formula | Name |
|---|---|---|---|
| \(\chem{HF(aq)}\) | Hydrofluoric acid | \(\chem{H_2SO_4(aq)}\) | Sulfuric acid |
| \(\chem{HCl(aq)}\) | Hydrochloric acid | \(\chem{H_2SO_3(aq)}\) | Sulfurous acid |
| \(\chem{HI(aq)}\) | Hydroiodic acid | \(\chem{HClO_4(aq)}\) | Perchloric acid |
| \(\chem{H_2S(aq)}\) | Hydrosulfuric acid | \(\chem{HClO_3(aq)}\) | Chloric acid |
| \(\chem{H_2CO_3(aq)}\) | Carbonic acid | \(\chem{HClO_2(aq)}\) | Chlorous acid |
| \(\chem{HNO_3(aq)}\) | Nitric acid | \(\chem{HClO(aq)}\) | Hypochlorous acid |
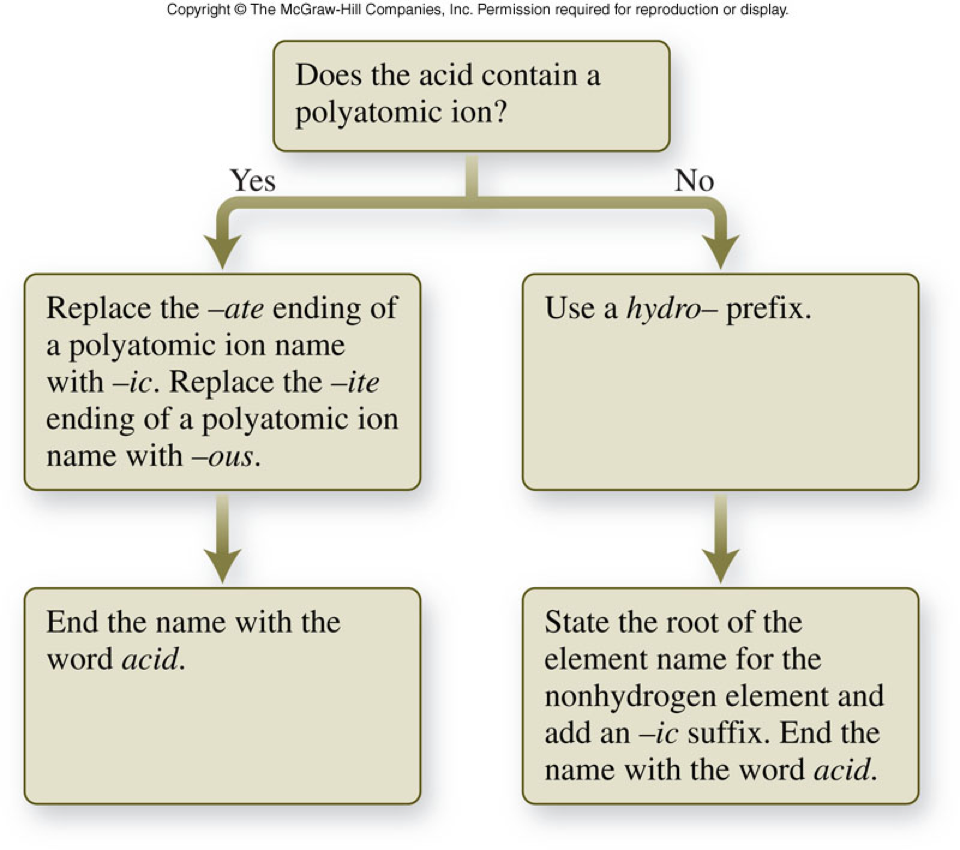
Naming Acids
- Since most acids have a hydrogen as their 1st element in the molecular formula, the acids are named according to the anion, not the cation (\(\chem{H^+}\)).
- Binary acids are named as hydro- followed by the root of the element (2nd element or anion) name with an –ic suffix and the word acid placed at the end of the name.
- Acids containing polyatomic ions (as the anion) are named by taking the root of the polyatomic ion name, replacing –ate with –ic or replacing –ite with –ous and adding the word acid at the end.
Summary of Naming Acids
Summary of Naming Compounds
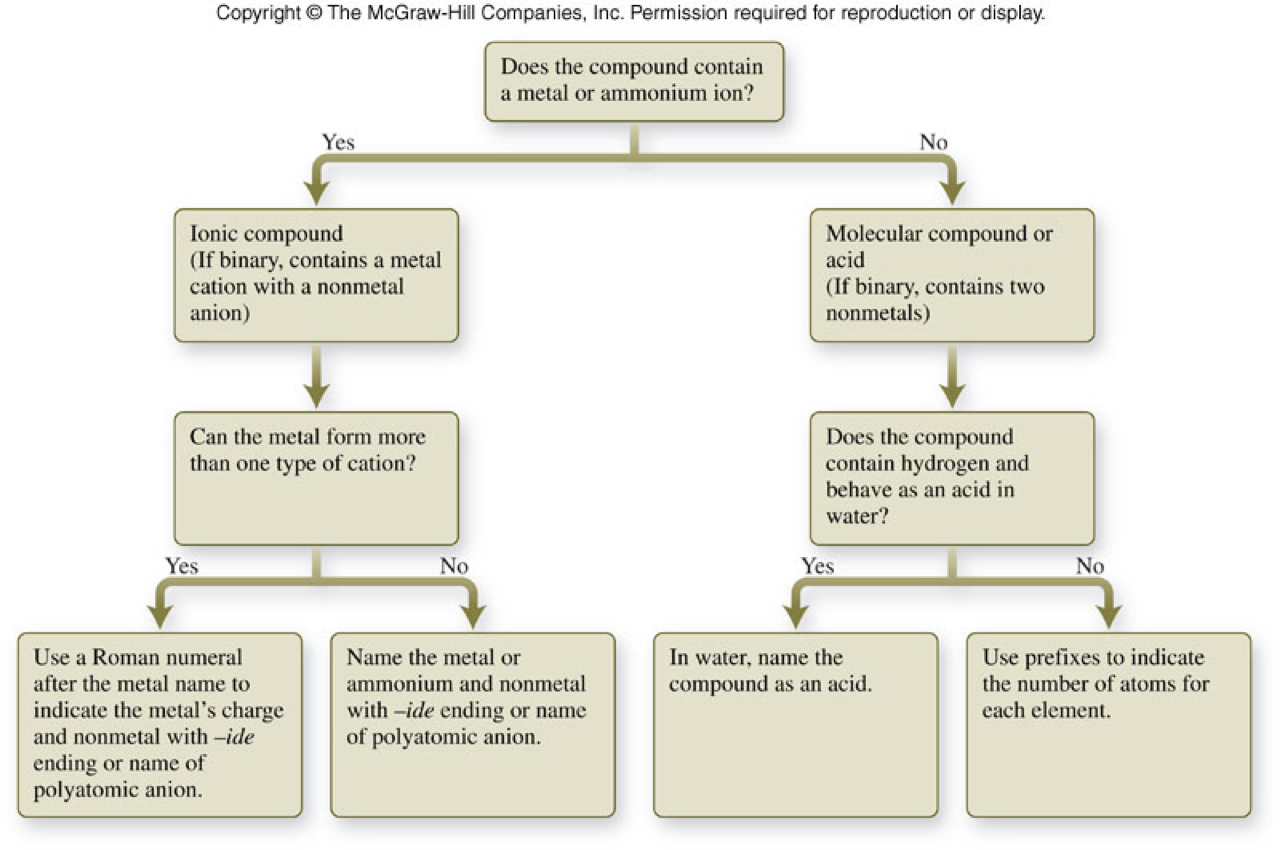
A General Summary of Naming Compounds
| Type of Compound | Naming |
|---|---|
| Ionic | Cation named first followed by anion |
| Molecular | 1st atom in formula (the element farther down or to the left in the periodic table) named 1st with the second element named as if it were an anion. Greek prefixes are used to designate the number of atoms in a molecule. |
| Acids | Binary acids are named as hydro- followed by the root of the element name with an –ic suffix and the word acid placed at the end of the name. Acids containing polyatomic ions are named by taking the root of the polyatomic ion name, replacing –ate with –ic or replacing –ite with –ous and adding the word acid at the end. |
/