Chapter 14
Oxidation-Reduction Reactions
Shaun Williams, PhD
What is an Oxidation-Reduction Reaction?
- A reaction in which electrons are transferred
- Also called redox reactions
- An example: \[ \chem{Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)} \] In this reaction, electrons are transferred from zinc to copper.

Oxidation-Reduction Reaction
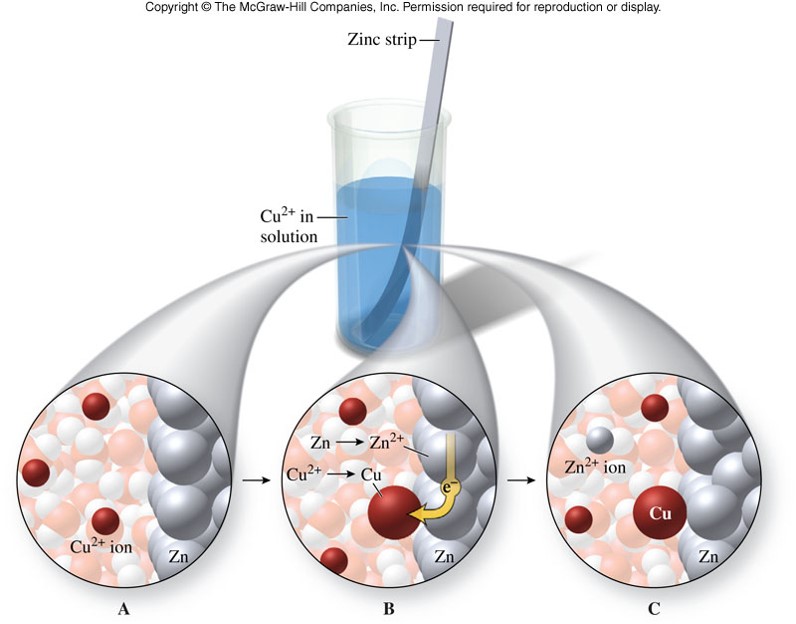
Oxidation and Reduction
- Oxidation - The process of losing one or more electrons
- Reduction - The process of gaining one or more electrons
- Oxidation and reduction are coupled reactions.
- Oxidation does not occur without reduction.
Oxidation and Reduction (cont.)
\[ \chem{\underset{\underset{0}\uparrow}{Zn}(s) + \underset{\underset{2+}\uparrow}{Cu}\underset{\underset{1-}\uparrow}{Cl_2}(aq) \rightarrow \underset{\underset{2+}\uparrow}{Zn}\underset{\underset{1-}\uparrow}{Cl_2}(aq) + \underset{\underset{0}\uparrow}{Cu}(s)} \]
- Only the reactants that change charge are involved in the redox reaction
- Zinc is oxidized because it changed charge from no charge (0) to +2, and thus lost two electrons.
- Copper is reduced because it changed charge from a +2 to no charge (0), and thus gained two electrons.
- Chlorine is not involved in the reaction because it did not change its charge.
Oxidizing and Reducing Agents
- Oxidizing agent
- A reactant that gains electrons and is reduced is the oxidizing agent because it accepts the electrons that are lost by the reactant that is oxidized.
- Reducing agent
- A reactant that loses electrons and is oxidized is the reducing agent because it provides electrons to the reactant that gets reduced.
Oxidizing and Reducing Agents (cont.)
\[ \chem{\underset{\underset{0}\uparrow}{Zn}(s) + \underset{\underset{2+}\uparrow}{Cu}\underset{\underset{1-}\uparrow}{Cl_2}(aq) \rightarrow \underset{\underset{2+}\uparrow}{Zn}\underset{\underset{1-}\uparrow}{Cl_2}(aq) + \underset{\underset{0}\uparrow}{Cu}(s)} \]
- Zinc is oxidized and loses two electrons to copper, making zinc the reducing agent.
- Copper is reduced and gains two electrons from zinc, making copper the oxidizing agent.
Oxidation Numbers
- A charge assigned to the atoms in any compound
- Also called oxidation states
- For ionic compounds, the oxidation number is the same as the ion's charge.
- We treat covalent compounds as if they’re ionic compounds, assigning oxidation numbers to elements using oxidation number rules.
- We use rules to assign oxidation numbers
- The rules are a hierarchy. The first rule that applies takes precedence over any subsequent rules that may apply.
Rules to Assign Oxidation Numbers
- The oxidation number of the atoms of an uncombined element is \(0\).
- The sum of the oxidation numbers of all atoms in a substance must equal the total charge: \(0\) for molecules, but the ionic charge for ions (including polyatomic ions).
- Fluorine has an oxidation number of \(1-\) in its compounds.
- Hydrogen has an oxidation number of \(1+\) unless it is combined with metals, where it has an oxidation number of \(1-\).
Rules to Assign Oxidation Numbers (cont.)
- The position of the element in the periodic table may be useful.
- Group 1 elements have oxidation numbers of \(1+\) in their compounds.
- Group 2 elements have oxidation numbers of \(2+\) in their compounds.
- Group 17 elements have oxidation numbers of \(1-\) unless combined with a more electronegative nonmetal.
- In binary compounds, Group 16 elements have oxidation numbers of \(2-\) unless combined with a more electronegative nonmetal.
- In binary compounds, Group 15 elements have oxidation numbers of \(3-\) unless combined with a more electronegative nonmetal.
- Oxygen has an oxidation number of \(2-\) except in peroxides (compounds containing the \(\chem{O_2^{2-}}\) ion) in which its oxidation number is \(1-\).
Batteries
Electricity and Batteries
- Electricity from a battery is a flow of electrons released from an oxidation-reduction reaction.
- Example (the zinc-mercury battery used in watches and calculators): \[ \chem{Zn(s) + HgO(s) \rightarrow Hg(l) + ZnO(s)} \]
- A barrier in the watch separates the oxidation reaction from the reduction reaction, and a wire connects the two reactions so that the energy generated is not lost as heat.

Voltaic Cells
- A device that sets up a spontaneous chemical reaction to produce electricity by using a barrier to separate the oxidation and reduction reactions
- Are the operational parts of a battery
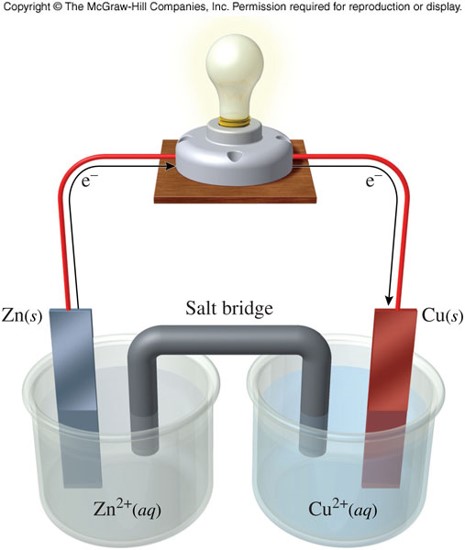
Oxidation and Reduction
- Half reactions represent either the oxidation or the reduction that occurs in separate compartments of a voltaic cell.
- Each compartment is called a half-cell.
- Oxidation half-reaction \[ \chem{Zn(s) \rightarrow Zn^{2+}(aq) + 2e^-} \]
- Reduction half-reaction \[ \chem{Cu^{2+}(aq) + 2e^- \rightarrow Cu(s)} \]
- Notice that because the electrons exist on either side of the arrow in the two reactions, they cancel out.
- The separation of the oxidation and reduction processes into half-cells harnesses the energy released from a chemical reaction to generate electricity.
The Parts of a Voltaic Cell
- Electrode
- A solid material that conducts electricity that must be present to provide a site for each half-reaction
- Commonly a metal immersed in an electrolyte solution containing a salt of the same metal
- Anode - The electrode at which oxidation occurs
- Cathode - The electrode at which reduction occurs
- Electricity will not flow unless there is a complete circuit between the anode and the cathode. A salt bridge is used to complete the circuit.
- Salt bridge - Allows ions to flow so charge balance is maintained
A Grapical Voltaic Cells
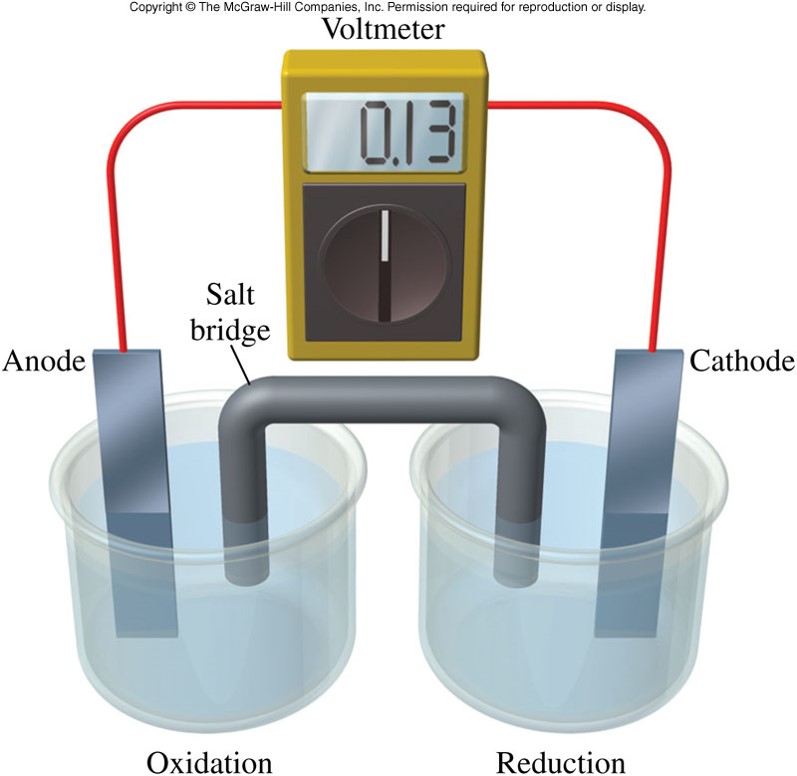
A Grapical Voltaic Cells - Action of the Salt Bridge
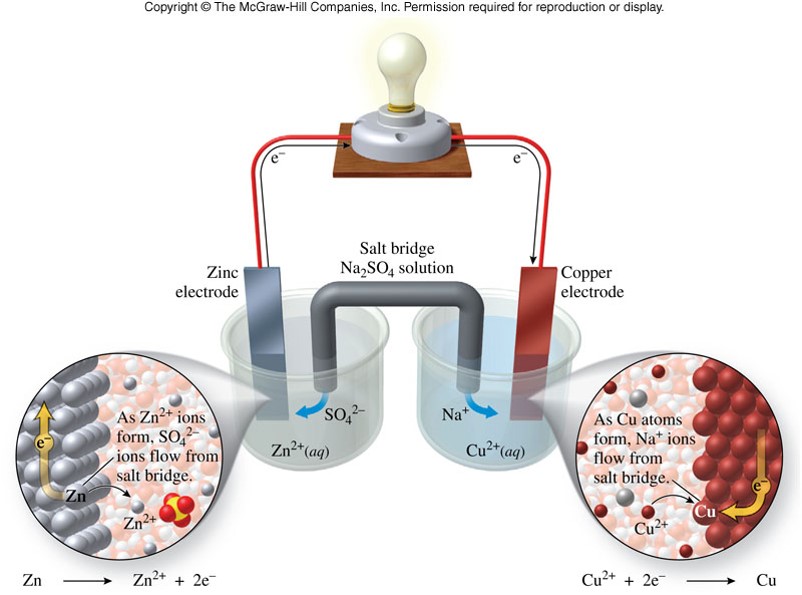
Batteries
- A series of connected voltaic cells that provides a portable source of electricity power
- The battery used in cars is a wet cell battery like copper and zinc–the lead storage battery: \[ \chem{Pb(s) + PbO_2(s) + 2H_2SO_4(aq) \rightarrow 2PbSO_4(s) + 2H_2O(l)} \] \[ \text{Anode: }\chem{Pb(s) + H_2SO_4(aq) \rightarrow PbSO_4(s) + 2H^+(aq) + 2e^-} \] \[ \text{Cathode: }\begin{array}{l} \chem{PbO_2(s) + H_2SO_4(aq) + 2H^+(aq) + 2e^-} \\ \chem{\phantom{aaaaaaaaaaaa}\rightarrow 2PbSO_4(s) + 2H_2O(l)} \end{array} \]
Balancing Simple Oxidation-Reduction Equations
- The reaction between silver nitrate and copper can be written simply as: \[ \chem{Cu(s) + Ag^+(aq) \rightarrow Cu^{2+}(aq) + Ag(s)} \]
- Does this reaction seem balanced to you? Do the atoms balance? Does the charge balance?
- You probably noticed that the total of the charges of the reactants is \(1+\), and the total of the charges of the products is \(2+\). Electrons lost do not equal electrons gained.
- The reaction is NOT balanced.
Balancing Simple Reactions - Part 1
\[ \chem{Cu(s) + Ag^+(aq) \rightarrow Cu^{2+}(aq) + Ag(s)} \]
- Separating the reaction into half-reactions: \[ \text{Oxidation: }\chem{Cu(s) \rightarrow Cu^{2+}(aq) + 2e^-} \] \[ \text{Reduction: }\chem{Ag^+(aq) + 1e^- \rightarrow Cu(s)} \]
- The electrons lost must equal the electrons gained, so we need to multiply the reduction half-reaction by 2: \[ \chem{2\left[ Ag^+(aq) + 1 e^- \rightarrow Ag(s)\right]} \] \[ \chem{2Ag^+(aq) + 2 e^- \rightarrow 2Ag(s)} \]
Balancing Simple Reactions - Part 2
\[ \chem{Cu(s) + Ag^+(aq) \rightarrow Cu^{2+}(aq) + Ag(s)} \]
- Now we can add the two half-reactions: \[ \begin{array}{rcl} \chem{Cu(s)} & \rightarrow & \chem{Cu^{2+}(aq) + 2e^-} \\ \chem{+\;\;\;\; 2Ag^+(aq) + 2e^-} & \rightarrow & \chem{2Ag(s)} \\ \hline \chem{Cu(s)+2Ag^+(aq)} & \rightarrow & \chem{Cu^{2+}(aq)+2Ag(s)} \end{array} \]
- Notice that 2 electrons appear as a reactant and a product. They cancel, and the overall reaction is: \[ \chem{Cu(s)+2Ag^+(aq) \rightarrow Cu^{2+}(aq)+2Ag(s)} \]
Balancing Simple Reactions - Part 3
\[ \chem{Cu(s)+2Ag^+(aq) \rightarrow Cu^{2+}(aq)+2Ag(s)} \] Double-checking the numbers of atoms and the overall charge:
| Reactants | Products | |
|---|---|---|
| \(\chem{Cu}\) | 1 | 1 |
| \(\chem{Ag}\) | 2 | 2 |
| Charge | 2+ | 2+ |
Helpful Hints in Balancing Simple Reactions
- Some questions to guide you through the balancing process:
- Which substance is oxidized and which is reduced? To answer this question, assign oxidation numbers to all the elements in the reaction.
- What are the half-reactions for the oxidation and reduction processes? For each half-reaction,
- Which element changes in oxidation number?
- Can any spectator ions be ignored until the final balancing step?
- How many electrons must be added to the appropriate side of the equation to account for the change in oxidation number?
Helpful Hints in Balancing Simple Reactions (cont.)
- What factor can be used to multiply each coefficient in the balanced half-reactions to equalize the number of electrons gained or lost?
- When adding the two half-reactions, can any substances that are present in equal amounts on both sides of the equation be canceled out?
- After answering these questions, you should have a balanced overall equation.
- Make sure the sum of the atoms and the sum of the charges equal on either side of the equation.
Balancing Complex Oxidation-Reduction Equations
- What reactant is oxidized and what reactant is reduced? Assign oxidation numbers to all the elements in the reaction to make the determination.
- What are the half reactions for the oxidation and reduction processes? For each half-reaction, determine each of the following:
- Is the element that changes the oxidation number balanced?
- Can any spectator ion be ignored until the final balancing step?
Balancing Complex Equations - Part 2
- Continued
- How many electrons must be added to the appropriate side of the equation to account for the change in oxidation number?
- Is the charge balanced after adding electrons? If not, what ions are present to balance the charge? If the reaction occurs in base, add \(\chem{OH^-}\) ions. If the reaction occurs in acid, add \(\chem{H^+}\) ions.
- Do the hydrogen and oxygen atoms balance? If not, add water molecules to the appropriate side of the equation.
Balancing Complex Equations - Part 3
- What factor can be used to multiply each coefficient to equalize the number of electrons gained and lost?
- When adding the two half-reactions, are any substances present on both sides of the equation? If so, cancel out an appropriate number. If substances are present on both sides of the equation in unequal amounts, cancel out the appropriate amount of substances on each side.
Electrochemistry
- The study of the relationship between chemical reactions and electrical work
- 2 main branches of electrochemistry:
- The study of voltaic cells: deals with spontaneous, desirable chemical processes
- The study of electrolytic cells: deals with desirable chemical processes that do not occur on their own
- Spontaneous reaction
- A chemical or physical change that occurs by itself without outside intervention
- Does not need an outside source of energy to progress
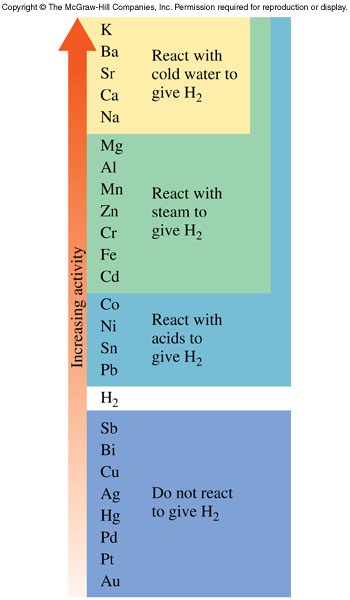
Activity Series
- An activity series predicts the relative strengths of metals as reducing agents and metal compounds as oxidizing agents
- As we go up the activity series, the metals become stronger reducing agents, which give up electrons more readily and are more reactive
- Metals in metal compounds increase in oxidizing strength as we move down the activity series
Making a Voltaic Cell from Household Products

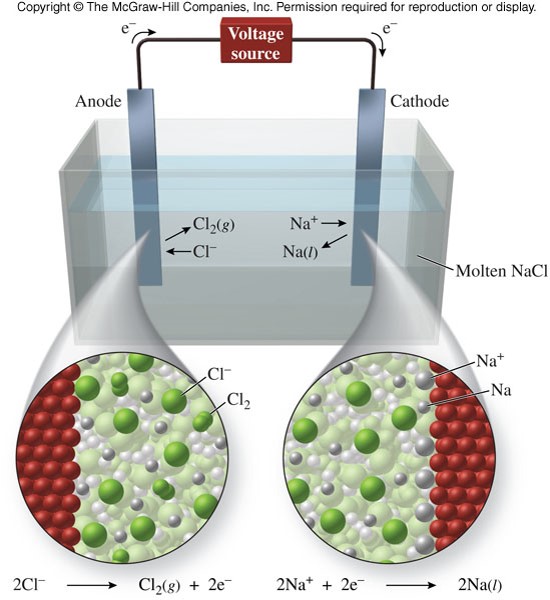
Electrolytic Cells
- An electrochemical cell through which electric current is passed to cause a nonspontaneous oxidation-reduction reaction to occur
- Electrolysis
- The process of passing electric current to cause a nonspontaneous redox reaction to occur
- The isolation of elements from their naturally occurring compounds
Electrolytic Cell - A Graphic
Corrosion Prevention
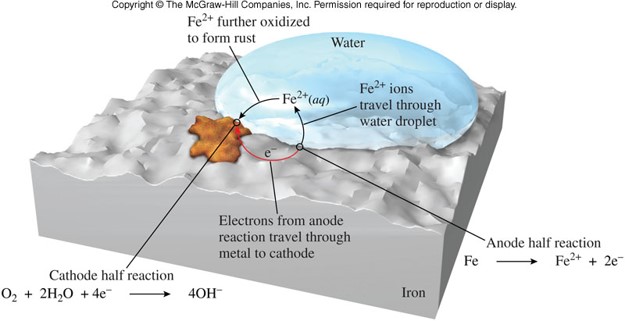
Corrosion
- Slow deterioration of metals due to interaction with the environment
- An oxidation-reduction reaction
- Familiar signs are:
- Red stains of rust on iron and steel
- Green coating on copper and brass
- Black coating on silver
/