Chapter 8
Advanced Theories of Covalent Bonding
Shaun Williams, PhD
Hybridization and the Localized Electron Model
Exercise 1
Draw the Lewis structure for methane, \(\chem{CH_4}\).
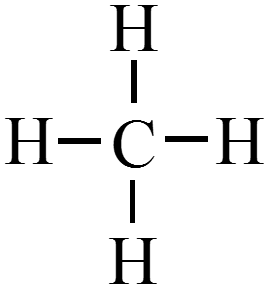
- What is the shape of a methane molecule? $$ \phantom{\text{tetrahedral}} $$
- What are the bond angles? $$ \phantom{109.5^\circ} $$
Hybridization and the Localized Electron Model
Exercise 1 - Answer
Draw the Lewis structure for methane, \(\chem{CH_4}\).

- What is the shape of a methane molecule? $$ \color{green} \text{tetrahedral} $$
- What are the bond angles? $$ \color{green} 109.5^\circ $$
Concept Check 1
What is the valence electron configuration of a carbon atom?
$$ \phantom{s^2p^2} $$
Why can't the bonding orbitals for methane be formed by an overlap of atomic orbitals?
Concept Check 1 - Answer
What is the valence electron configuration of a carbon atom?
$$ s^2p^2 $$
Why can't the bonding orbitals for methane be formed by an overlap of atomic orbitals?
Because this would lead to two different types of C-H bonds and we know that methane has four identical C-H bonds that are 109.5° apart from each other (not 90° from each other).
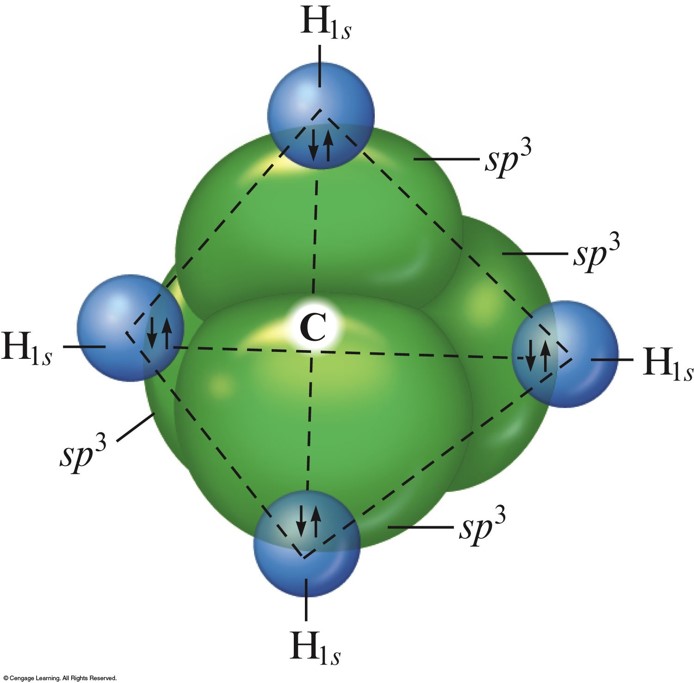
Bonding in Methane
- Assume that the carbon atom has four equivalent atomic orbitals, arranged tetrahedrally.
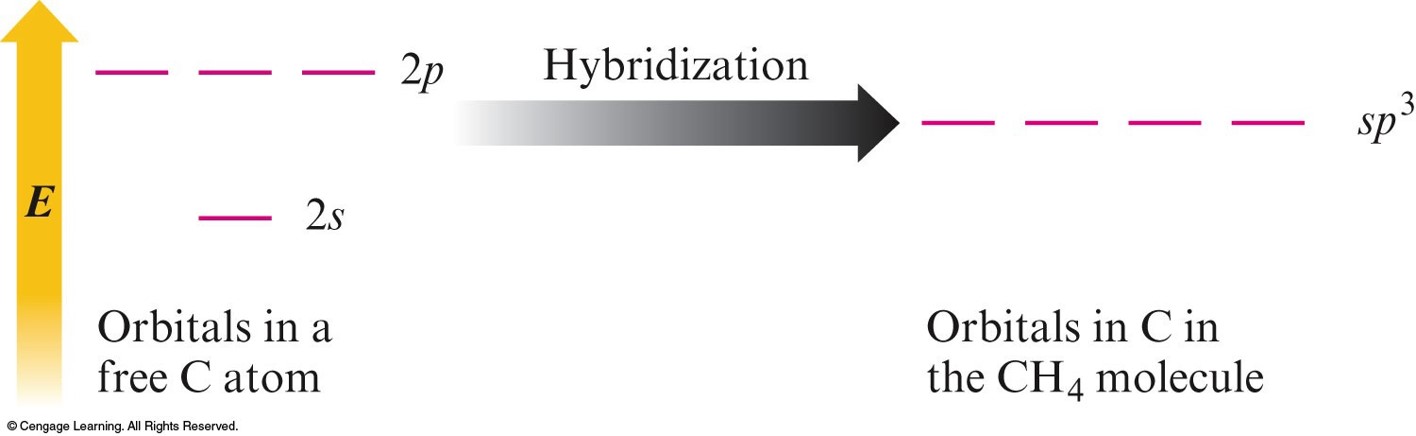
Hybridization
- Mixing of the native atomic orbitals to form special orbitals for bonding.
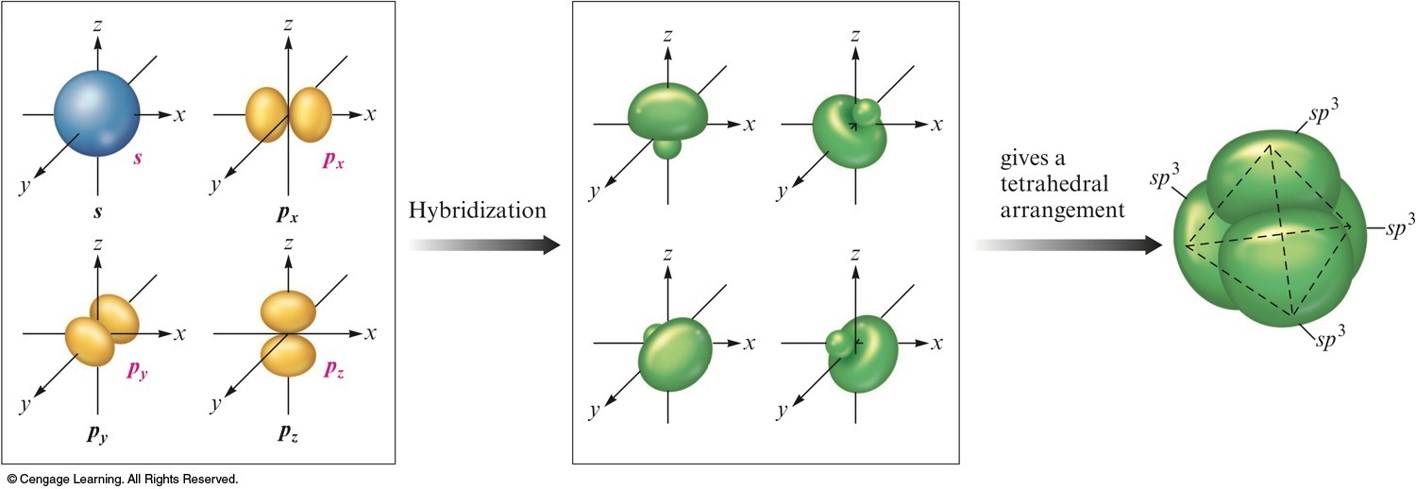
\(sp^3\) Hybridization
- Combination of one s and three p orbitals.
- Whenever a set of equivalent tetrahedral atomic orbitals is required by an atom, the localized electron model assumes that the atom adopts a set of \(sp^3\) orbitals; the atom becomes \(sp^3\) hybridized.
- The four orbitals are identical in shape.
An Energy-Level Diagram Showing the Formation of Four \(sp^3\) Orbitals
The Formation of \(sp^3\) Hybrid Orbitals
Exercise 2
Draw the Lewis structure for \(\chem{C_2H_4}\) (ethylene)?
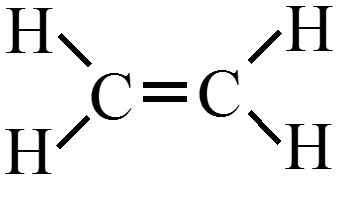
- What is the shape of an ethylene molecule? $$ \phantom{\text{trigonal planar around each carbon atom}} $$
- What are the approximate bond angles around the carbon atoms? $$ \phantom{120^\circ} $$
Exercise 2 - Answer
Draw the Lewis structure for \(\chem{C_2H_4}\) (ethylene)?

- What is the shape of an ethylene molecule? $$ \color{green} \text{trigonal planar around each carbon atom} $$
- What are the approximate bond angles around the carbon atoms? $$ \color{green} 120^\circ $$
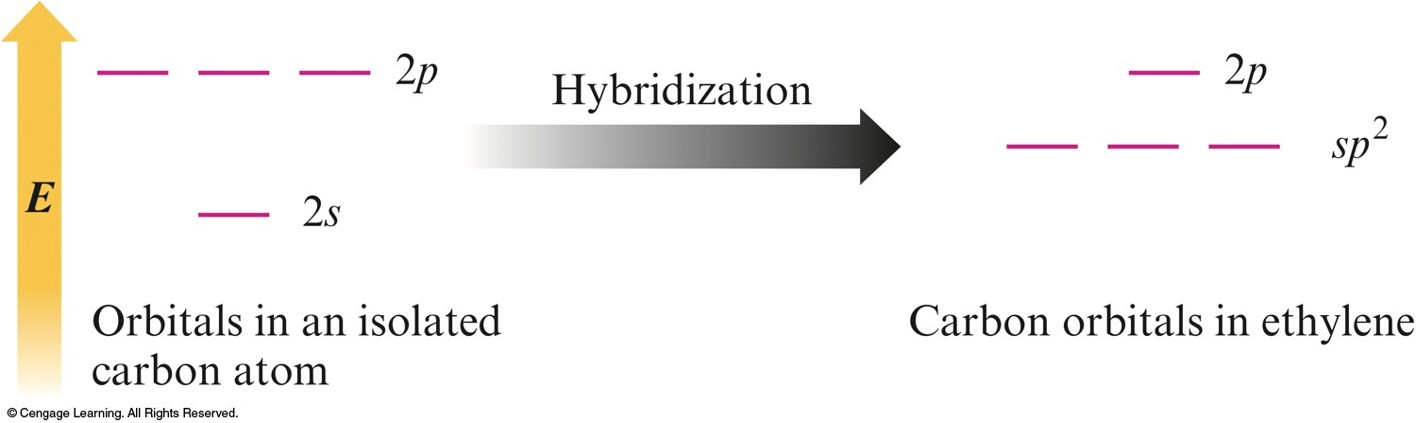
\(sp^2\) Hybridization
- Combination of one \(s\) and two \(p\) orbitals.
- Gives a trigonal planar arrangement of atomic orbitals.
- One \(p\) orbital is not used.
- Oriented perpendicular to the plane of the \(sp^2\) orbitals.
Sigma (\(\sigma\)) Bond
- Electron pair is shared in an area centered on a line running between the atoms.
Pi (\(\pi\)) Bond
- Forms double and triple bonds by sharing electron pair(s) in the space above and below the \(\sigma\) bond.
- Uses the unhybridized p orbitals.
An Orbital Energy-Level Diagram for \(sp^2\) Hybridization
The Hybridization of the \(s\), \(p_x\), and \(p_y\) Atomic Orbitals
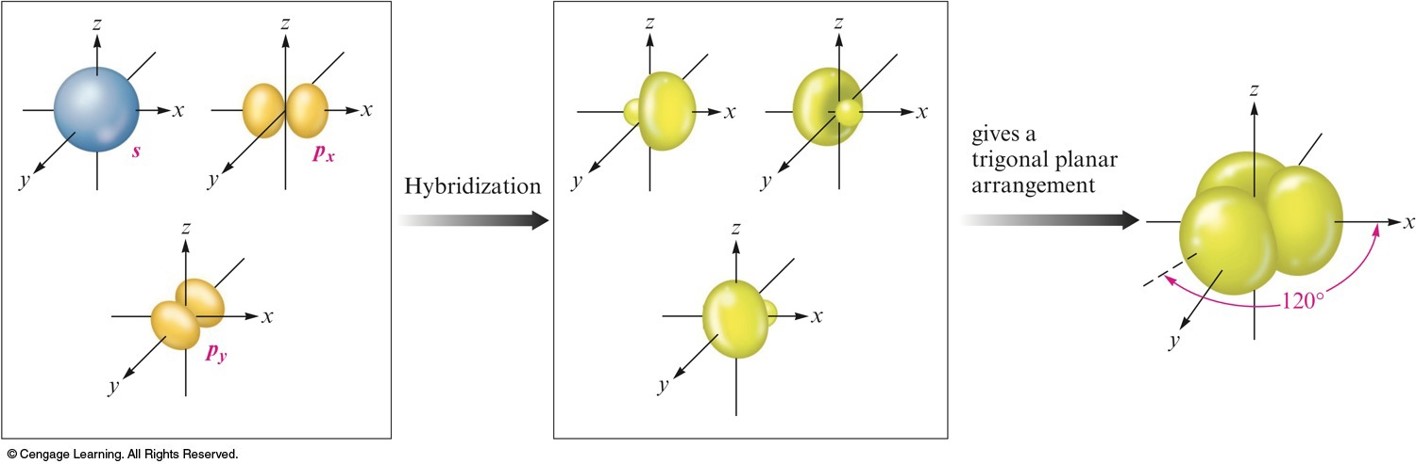
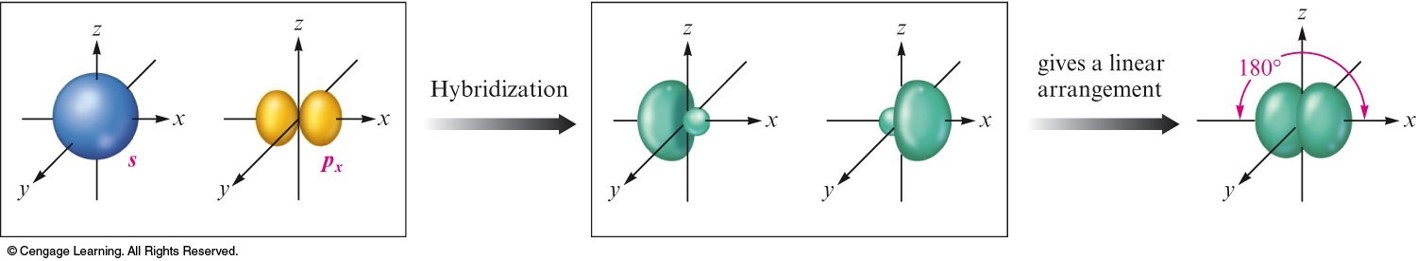
\(sp\) Hybridization
- Combination of one \(s\) and one \(p\) orbital.
- Gives a linear arrangement of atomic orbitals.
- Two \(p\) orbitals are not used.
- Needed to form the \(\pi\) bonds.
The Orbital Energy-Level Diagram for the Formation of \(sp\) Hybrid Orbitals on Carbon
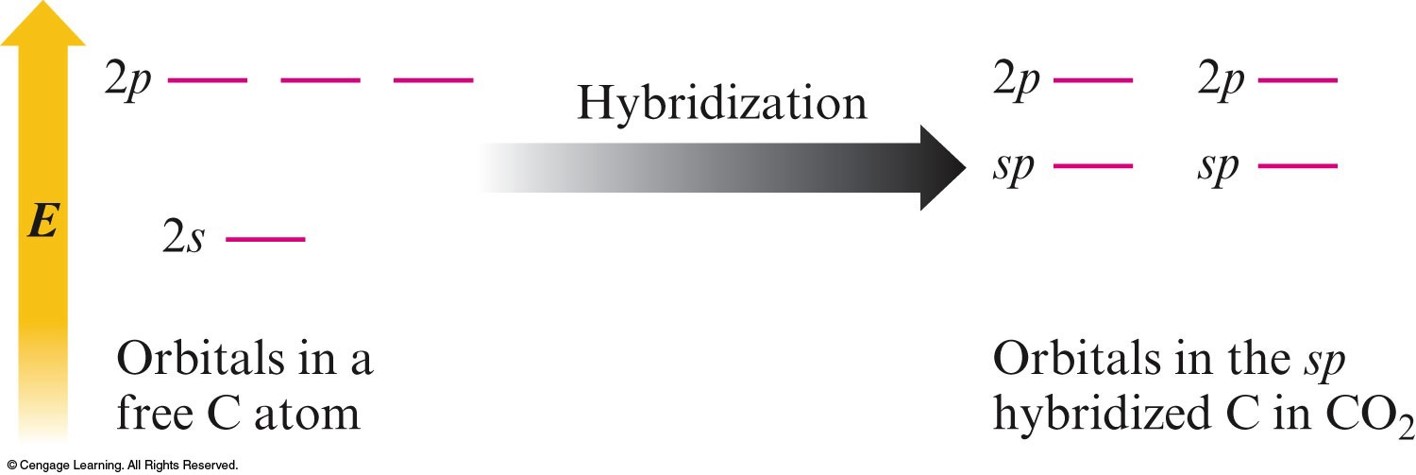
When One \(s\) Orbital and One \(p\) Orbital are Hybridized, a Set of Two \(sp\) Orbitals Oriented at \(180^\circ\) Results
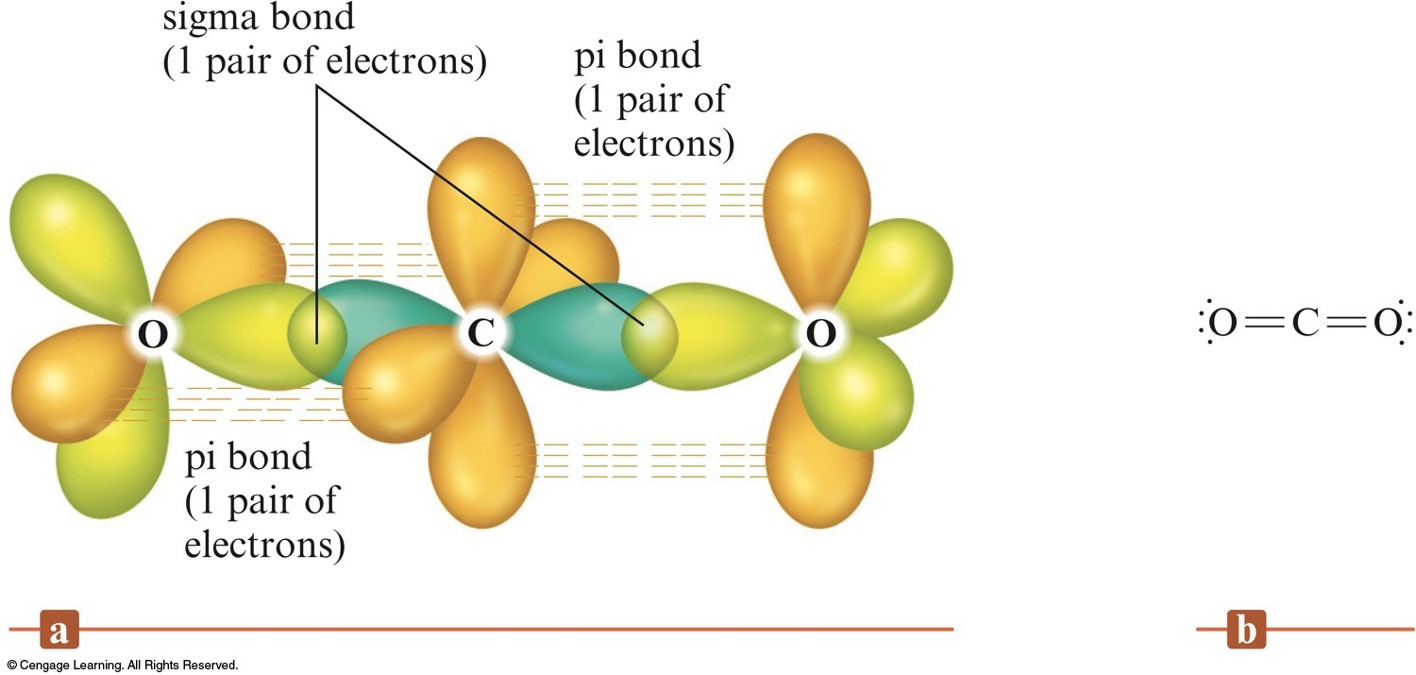
The Orbitals for \(\chem{CO_2}\)
Exercise 3
Draw the Lewis structure for \(\chem{PCl_5}\)?
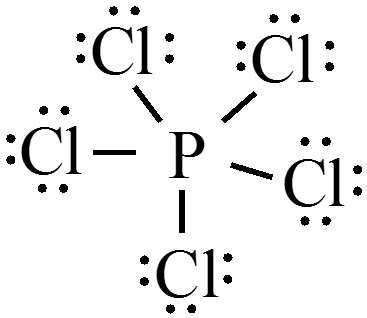
- What is the shape of phosphorus pentachloride? $$ \phantom{\text{trigonal bipyramidal}} $$
- What are the bond angles? $$ \phantom{90^\circ \text{ and } 120^\circ} $$
Exercise 3 - Answer
Draw the Lewis structure for \(\chem{PCl_5}\)?

- What is the shape of phosphorus pentachloride? $$ \color{green} \text{trigonal bipyramidal} $$
- What are the bond angles? $$ \color{green} 90^\circ \text{ and } 120^\circ $$
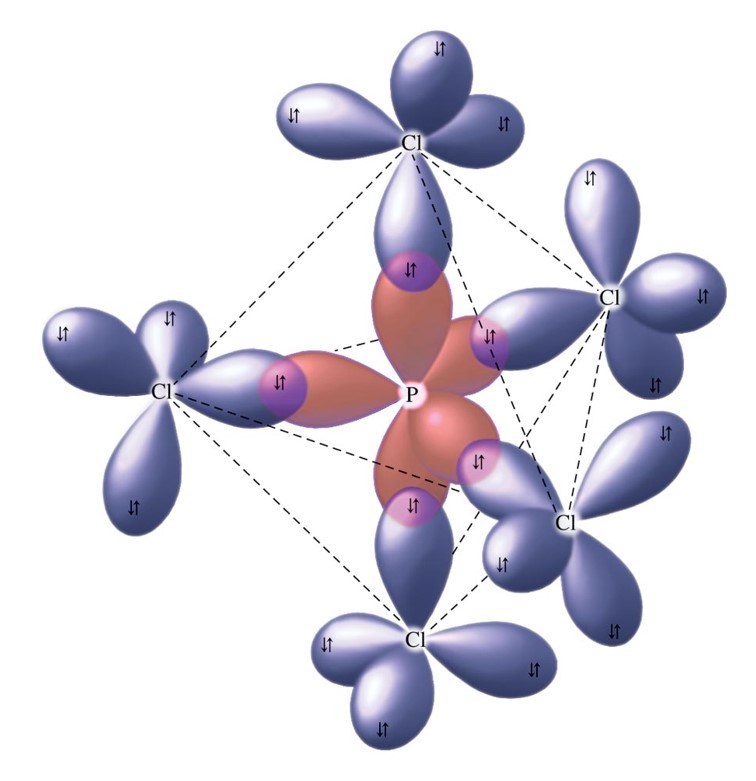
\(dsp^3\) Hybridization
- Combination of one \(d\), one \(s\), and three \(p\) orbitals.
- Gives a trigonal bipyramidal arrangement of five equivalent hybrid orbitals.
The Orbitals Used to Form the Bonds in \(\chem{PCl_5}\)
Exercise 3
Draw the Lewis structure for \(\chem{XeF_4}\)?
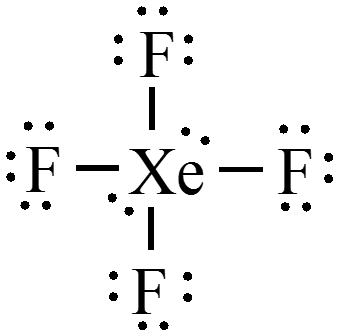
- What is the shape of an xenon tetrafluoride? $$ \phantom{\text{octahedral}} $$
- What are the bond angles? $$ \phantom{90^\circ \text{ and } 180^\circ} $$
Exercise 3 - Answer
Draw the Lewis structure for \(\chem{XeF_4}\)?

- What is the shape of an xenon tetrafluoride? $$ \color{green} \text{octahedral} $$
- What are the bond angles? $$ \color{green} 90^\circ \text{ and } 180^\circ $$
\(d^2sp^3\) Hybridization
- Combination of two \(d\), one \(s\), and three \(p\) orbitals.
- Gives an octahedral arrangement of six equivalent hybrid orbitals.
How is the Xenon Atom in \(\chem{XeF_4}\) Hybridized
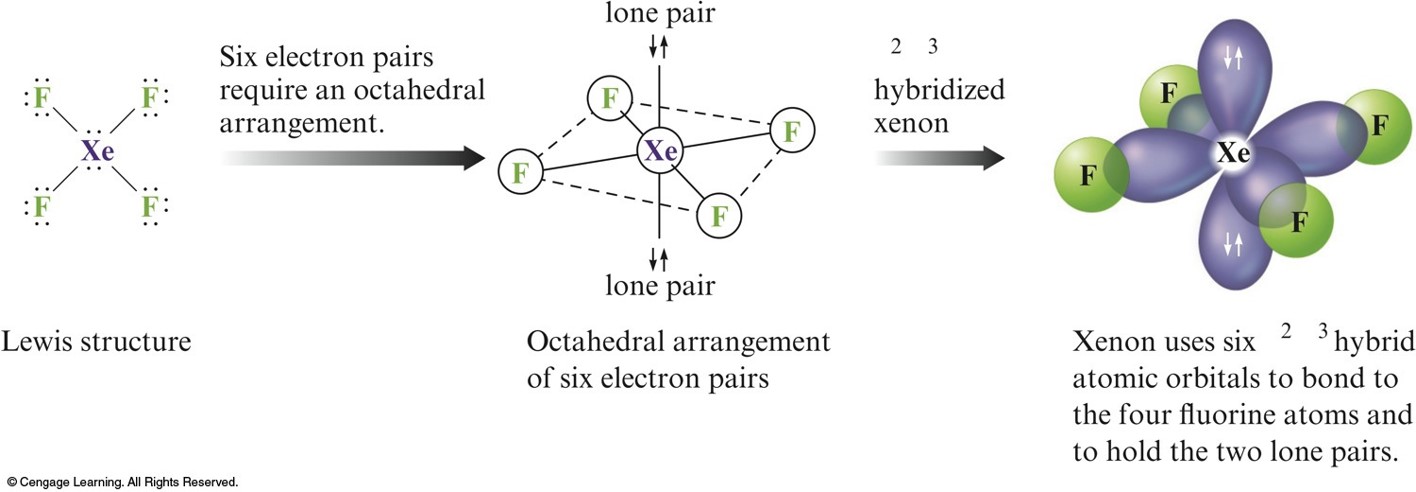
Using the Localized Electron Model
- Draw the Lewis structure(s).
- Determine the arrangement of electron pairs using the VSEPR model.
- Specify the hybrid orbitals needed to accommodate the electron pairs.
The Molecular Orbital Model
- Regards a molecule as a collection of nuclei and electrons, where the electrons are assumed to occupy orbitals much as they do in atoms, but having the orbitals extend over the entire molecule.
- The electrons are assumed to be delocalized rather than always located between a given pair of atoms.
- The electron probability of both molecular orbitals is centered along the line passing through the two nuclei.
- Sigma (\(\sigma\)) molecular orbitals (MOs)
- In the molecule only the molecular orbitals are available for occupation by electrons.
Combination of Hydrogen 1s Atomic Orbitals to form MOs
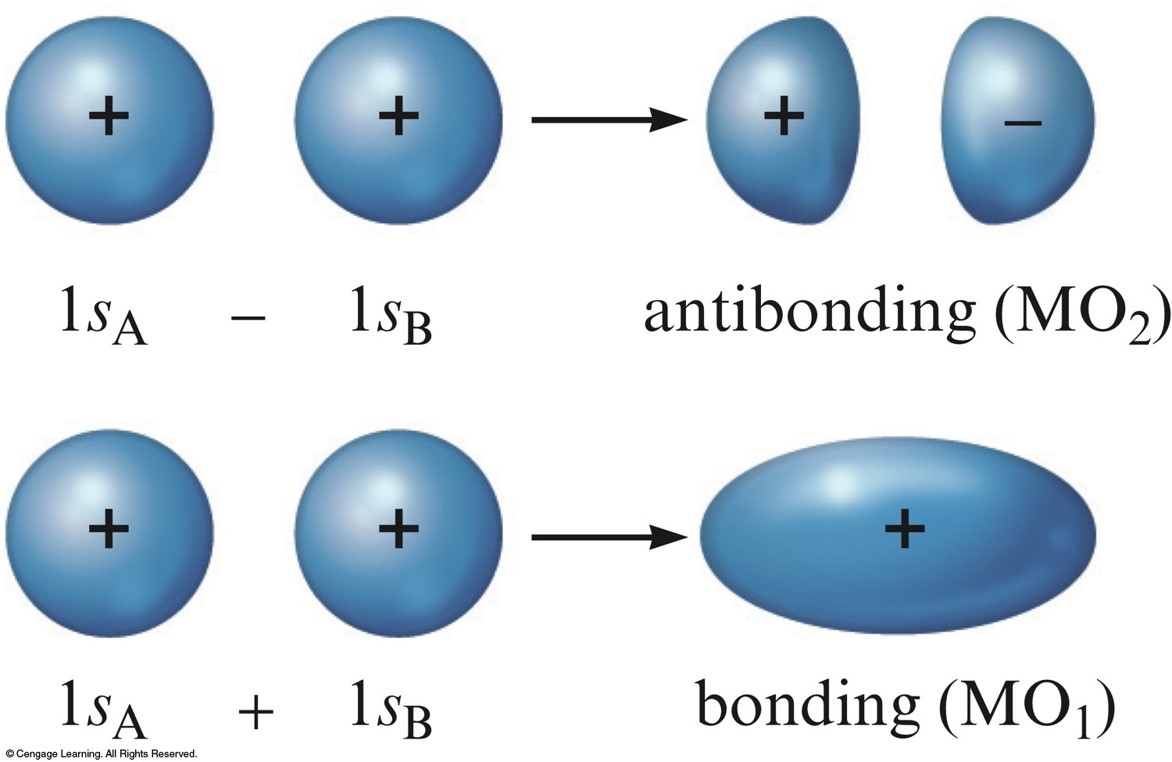
Relative Stabilities
- \(\chem{MO_1}\) is lower in energy than the \(s\) orbitals of free atoms, while \(\chem{MO_2}\) is higher in energy than the \(s\) orbitals.
- Bonding molecular orbital – lower in energy
- Antibonding molecular orbital – higher in energy
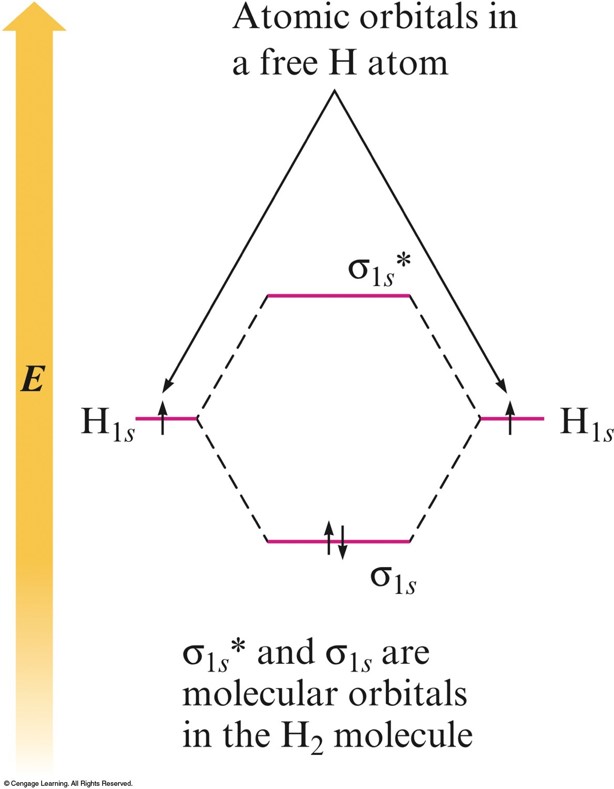
MO Energy-Level Diagram for the \(\chem{H_2}\) Molecule

Description of Bonding
- The molecular orbital model produces electron distributions and energies that agree with our basic ideas of bonding.
- The labels on molecular orbitals indicate their symmetry (shape), the parent atomic orbitals, and whether they are bonding or antibonding.
- Molecular electron configurations can be written in much the same way as atomic electron configurations.
- Each molecular orbital can hold 2 electrons with opposite spins.
- The number of orbitals are conserved.
Bond Order
- Larger bond order means greater bond strength. $$ \text{Bond order} = \frac{\left( \text{# of bonding }e^- \right) - \left( \text{# of antibonding }e^- \right)}{2} $$
Example: \(\chem{H_2}\)
$$ \text{Bond order}=\frac{2-0}{2} = 1 $$
Example: \(\chem{H_2^-}\)
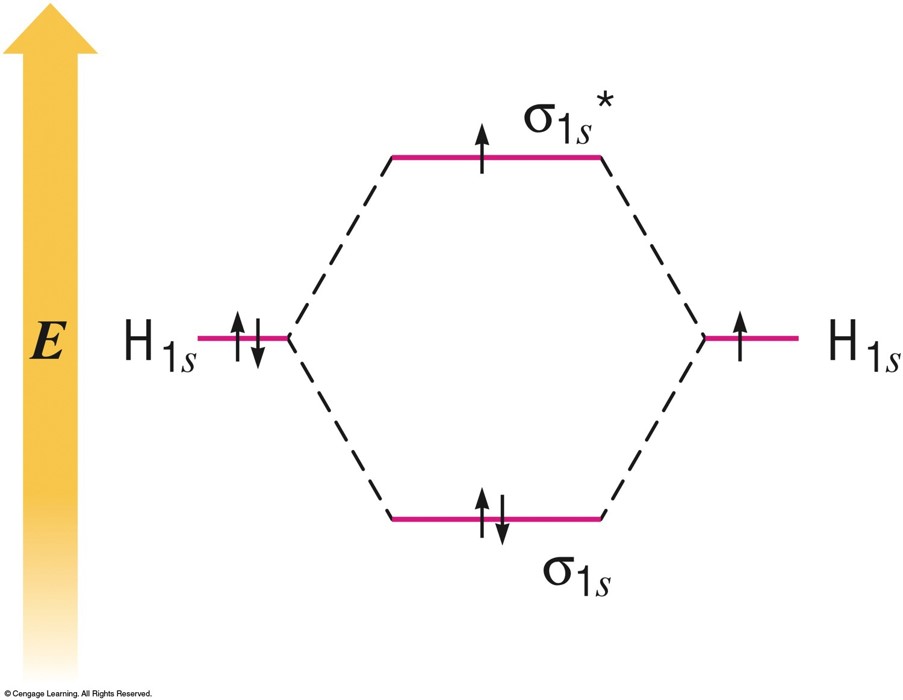
$$ \text{Bond order}=\frac{2-1}{2} = \frac{1}{2} $$
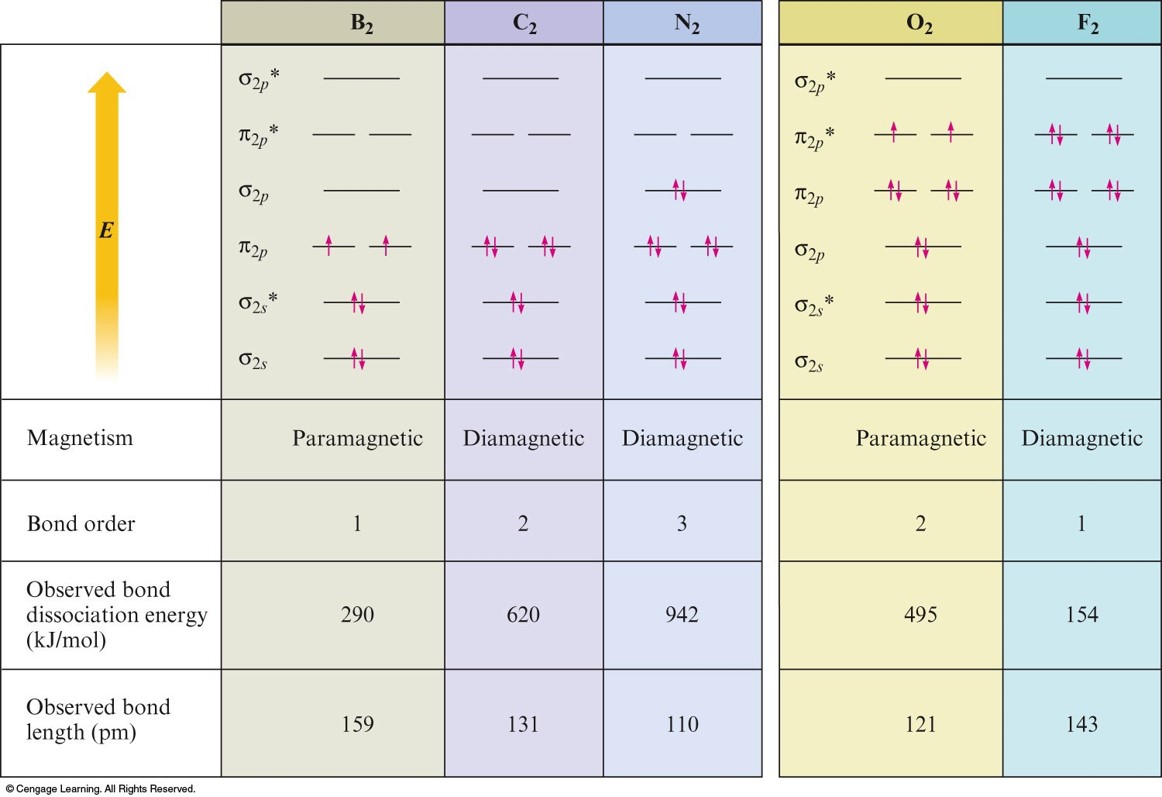
Bonding on Homonuclear Diatomic Molecules
Homonuclear Diatomic Molecules
- Composed of 2 identical atoms.
- Only the valence orbitals of the atoms contribute significantly to the molecular orbitals of a particular molecule.
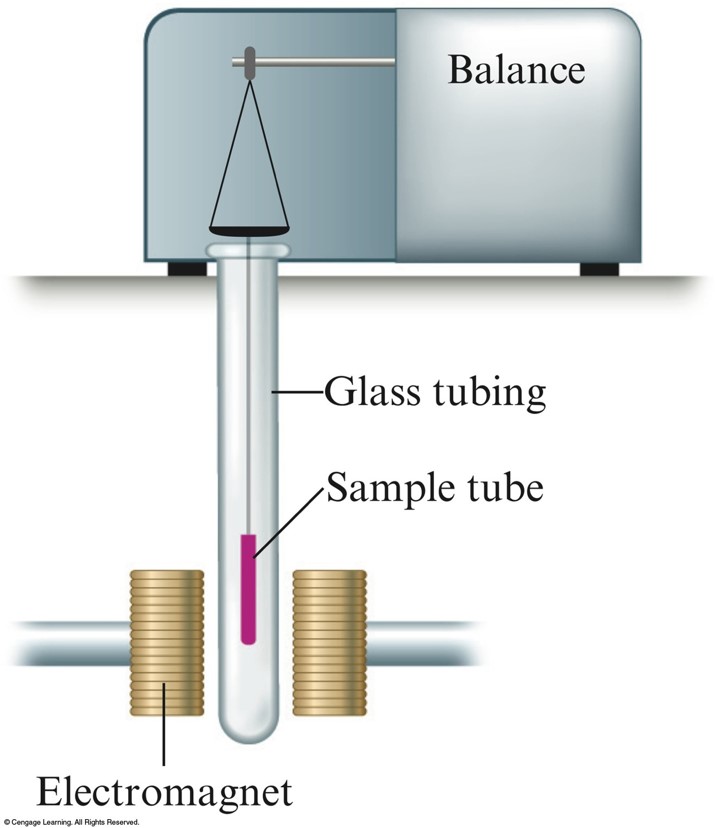
Paramagnetism
- Paramagnetism – substance is attracted into the inducing magnetic field.
- Unpaired electrons (\(\chem{O_2}\))
- Diamagnetism – substance is repelled from the inducing magnetic field.
- Paired electrons (\(\chem{N_2}\))
Apparatus Used to Measure the Paramagnetism of a Sample
Molecular Orbital Summary of Second Row Diatomic Molecules
Bonding in Heteronuclear Diatomic Molecules
Heteronuclear Diatomic Molecules
- Composed of 2 different atoms.
Heteronuclear Diatomic Molecule: \(\chem{HF}\)
- The \(2p\) orbital of fluorine is at a lower energy than the \(1s\) orbital of hydrogen because fluorine binds its valence electrons more tightly.
- Electrons prefer to be closer to the fluorine atom.
- Thus the \(2p\) electron on a free fluorine atom is at a lower energy than the \(1s\) electron on a free hydrogen atom.
Orbital Energy-Level Diagram for the \(\chem{HF}\) Molecule
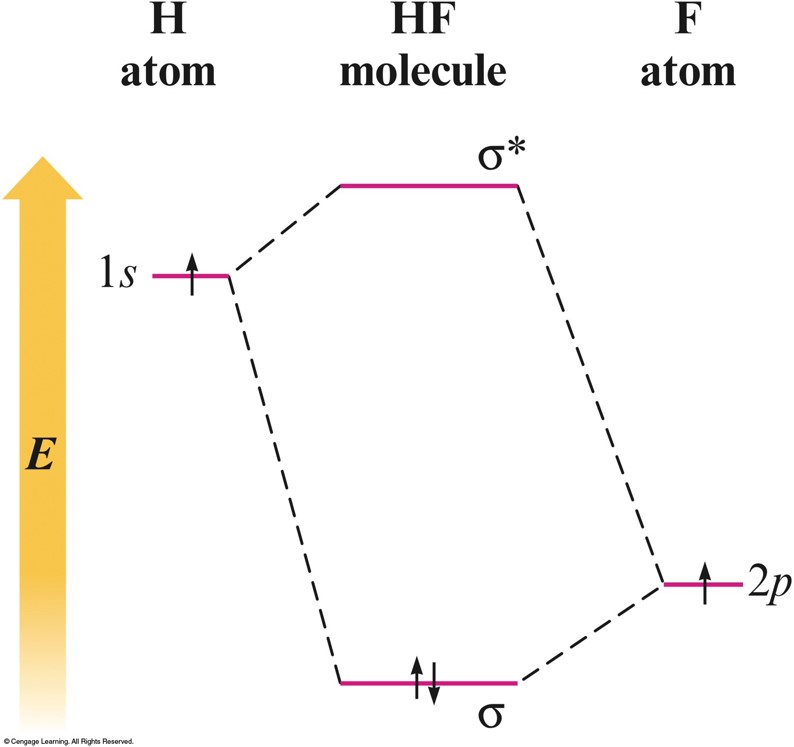
- The diagram predicts that the \(\chem{HF}\) molecule should be stable because both electrons are lowered in energy relative to their energy in the free hydrogen and fluorine atoms, which is the driving force for bond formation.
Heteronuclear Diatomic Molecule: HF (cont.)
- The \(\sigma\) molecular orbital containing the bonding electron pair shows greater electron probability close to the fluorine.
- The electron pair is not shared equally.
- This causes the fluorine atom to have a slight excess of negative charge and leaves the hydrogen atom partially positive.
- This is exactly the bond polarity observed for \(\chem{HF}\).
Combining the Localized Electron and Molecular Orbital Models
Delocalization
- Describes molecules that require resonance.
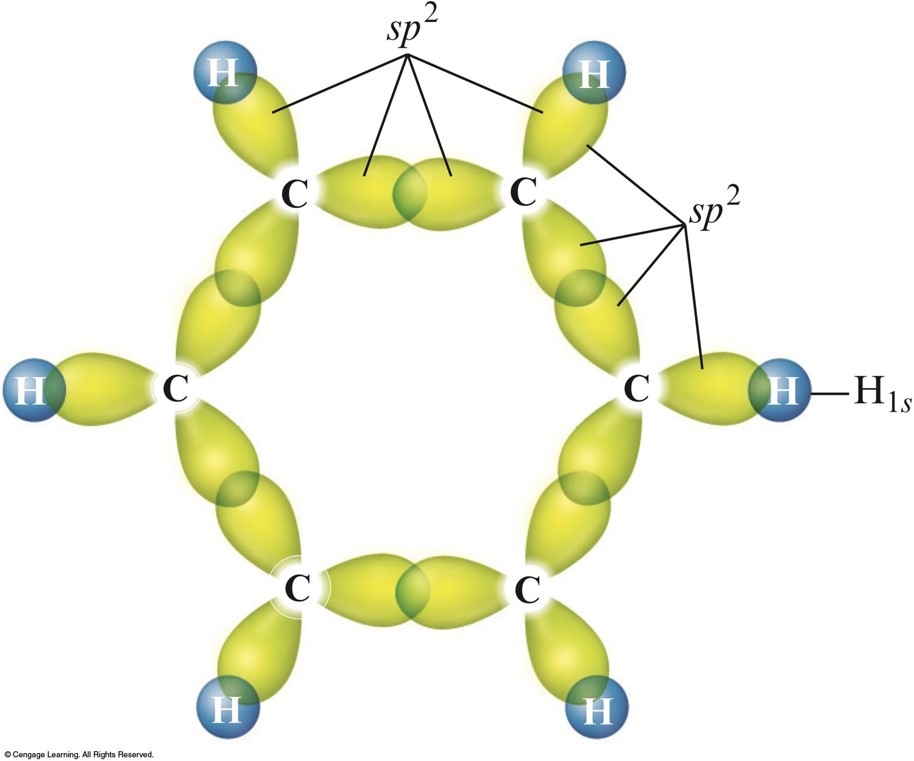
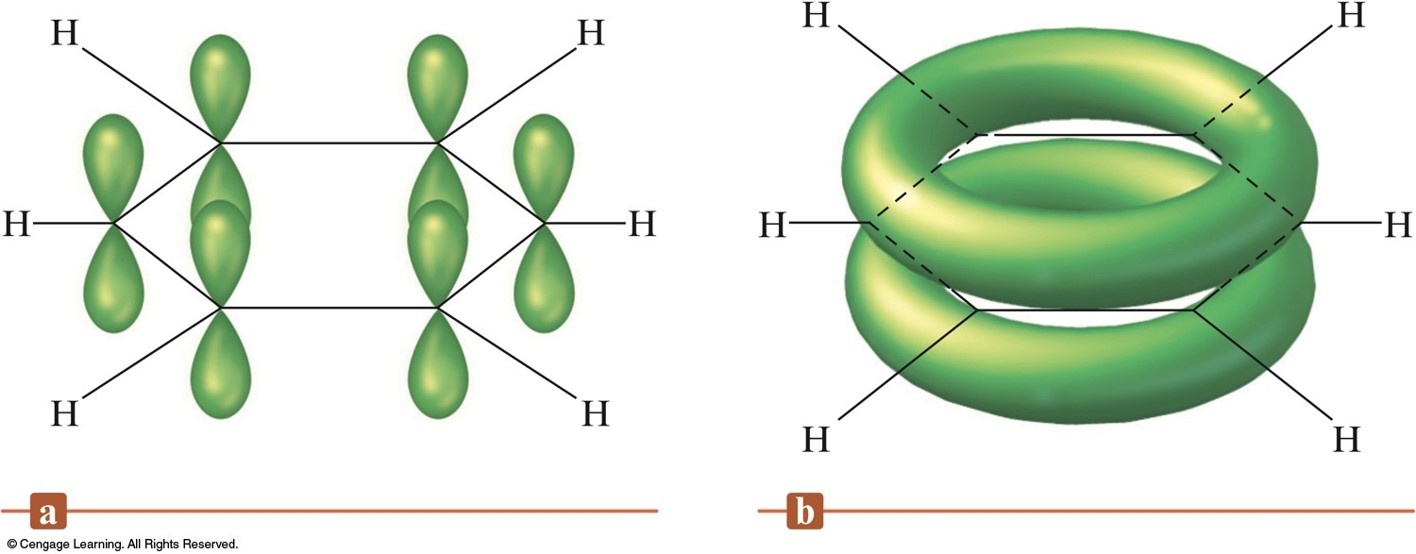
- In molecules that require resonance, it is the \(\pi\) bonding that is most clearly delocalized, the \(\sigma\) bonds are localized.
- \(p\) orbitals perpendicular to the plane of the molecule are used to form \(\pi\) molecular orbitals.
- The electrons in the \(\pi\) molecular orbitals are delocalized above and below the plane of the molecule.
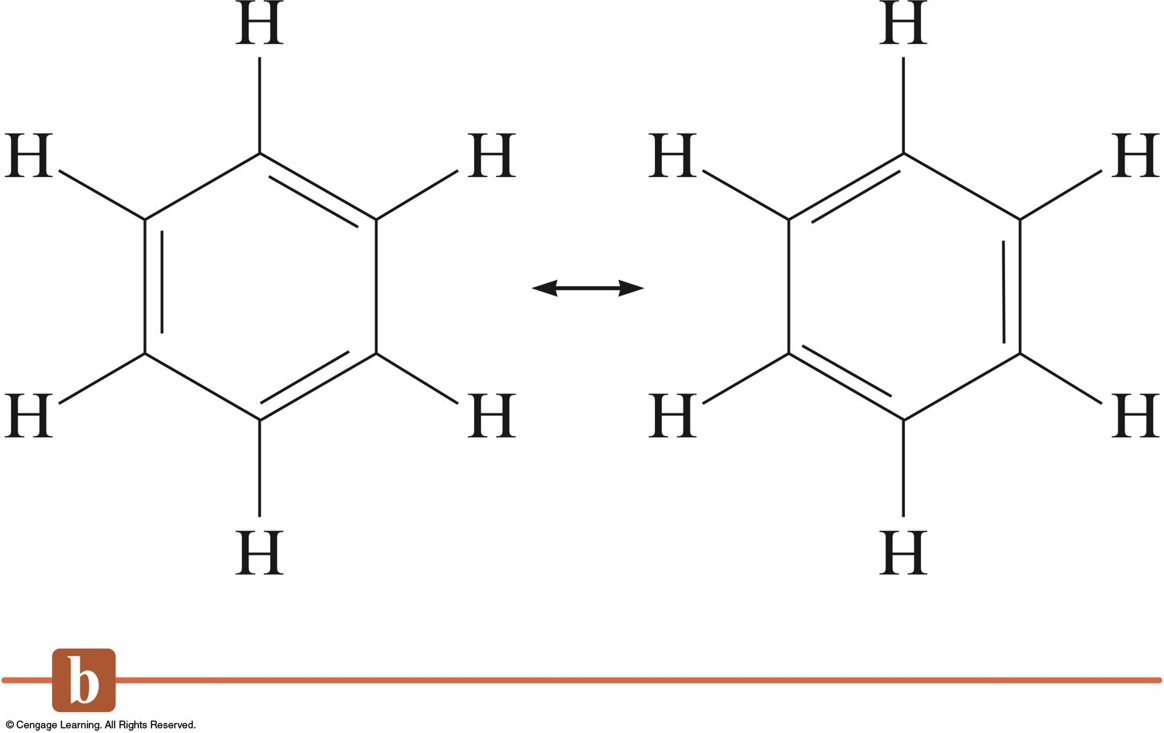
Resonance in Benzene
The Sigma System for Benzene
The Pi System for Benzene
/