Chapter 4
Types of Chemical Reactions and Solution Stoichiometry
Shaun Williams, PhD
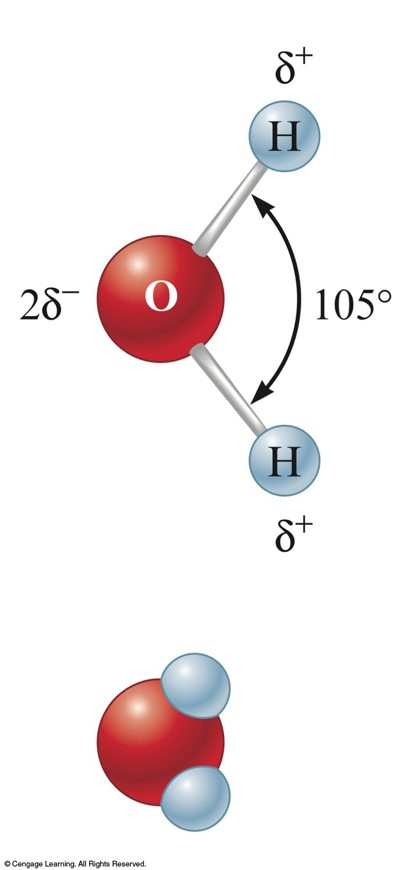
Water, the Common Solvent
Water
- One of the most important substances on Earth.
- Can dissolve many different substances.
- A polar molecule because of its unequal charge distribution.
The Nature of Aqueous Solutions: Strong and Weak Electrolytes
Nature of Aqueous Solutions
- Solute – substance being dissolved.
- Solvent – liquid water.
- Electrolyte – substance that when dissolved in water produces a solution that can conduct electricity.
Electrolytes
- Strong Electrolytes – conduct current very efficiently (bulb shines brightly). Completely ionized in water.
- Weak Electrolytes – conduct only a small current (bulb glows dimly). A small degree of ionization in water.
- Nonelectrolytes – no current flows (bulb remains unlit). Dissolves but does not produce any ions.
The Composition of Solutions
Chemical Reactions of Solutions
- We must know:
- The nature of the reaction.
- The amounts of chemicals present in the solutions.
Molarity
- Molarity (M) = moles of solute per volume of solution in liters: $$ \chem{M} = \text{Molarity} = \frac{\text{moles of solute}}{\text{liters of solution}} $$ $$ 3\,\chem{M}\,\chem{HCl} = \frac{6\,\text{moles of HCl}}{2\,\text{liters of solution}} $$
Exercise 1
A 500.0-g sample of potassium phosphate is dissolved in enough water to make 1.50 L of solution. What is the molarity of the solution?
Exercise 1 - Answer
A 500.0-g sample of potassium phosphate is dissolved in enough water to make 1.50 L of solution. What is the molarity of the solution?
$$ 1.57\,\chem{M} $$Concentration of Ions
- For a \(0.25\,\chem{M}\,\chem{CaCl_2}\) solution:
$$ \chem{CaCl_2 \rightarrow Ca^{2+} + 2Cl^-} $$
- \(\chem{Ca^{2+}}:\,1\times 0.25\,\chem{M} = 0.25\,\chem{M}\,\chem{Ca^{2+}}\)
- \(\chem{Cl^{-}}:\,2\times 0.25\,\chem{M} = 0.50\,\chem{M}\,\chem{Cl^{-}}\)
Notice
- The solution with the greatest number of ions is not necessarily the one in which:
- the volume of the solution is the largest.
- the formula unit has the greatest number of ions.
Dilution
- The process of adding water to a concentrated or stock solution to achieve the molarity desired for a particular solution.
- Dilution with water does not alter the numbers of moles of solute present.
- Moles of solute before dilution = moles of solute after dilution $$ M_1V_1 = M_2V_2 $$
Types of Chemical Reactions
- Precipitation Reactions
- Acid–Base Reactions
- Oxidation–Reduction Reactions
Precipitation Reactions
Precipitation Reaction
- A double displacement reaction in which a solid forms and separates from the solution.
- When ionic compounds dissolve in water, the resulting solution contains the separated ions.
- Precipitate – the solid that forms.
\( \chem{Ba\left(NO_3\right)_2(aq)+K_2CrO_4(aq)\rightarrow 2KNO_3(aq) + BaCrO_4(s)} \)
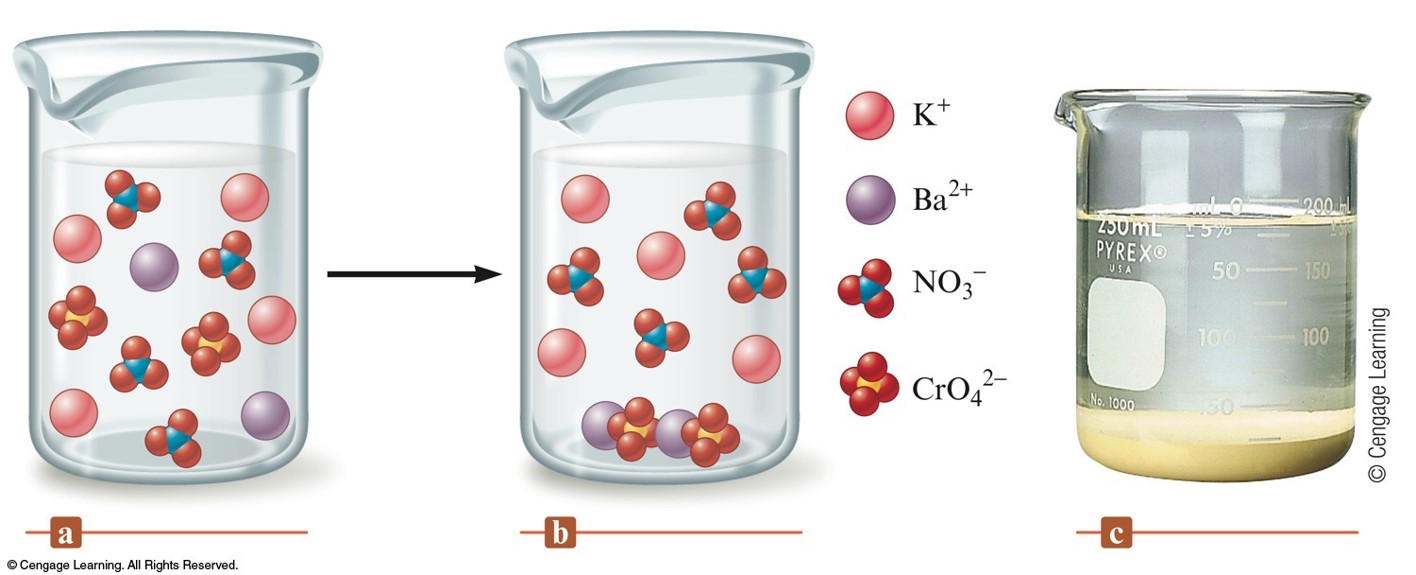
Precipitates
- Soluble – solid dissolves in solution; (aq) is used in reaction equation.
- Insoluble – solid does not dissolve in solution; (s) is used in reaction equation.
- Insoluble and slightly soluble are often used interchangeably.
Simple Rules for Solubility
- Most nitrate (\(\chem{NO_3^-}\)) salts are soluble.
- Most alkali metal (group 1A) salts and \(\chem{NH_4^+}\) are soluble.
- Most \(\chem{Cl^-}\), \(\chem{Br^-}\), and \(\chem{I^-}\) salts are soluble (except \(\chem{Ag^+}\), \(\chem{Pb^{2+}}\), \(\chem{Hg_2^{2+}}\)).
- Most sulfate salts are soluble (except \(\chem{BaSO_4}\), \(\chem{PbSO_4}\), \(\chem{Hg_2SO_4}\), \(\chem{CaSO_4}\)).
- Most \(\chem{OH^-}\) are only slightly soluble (\(\chem{NaOH}\), \(\chem{KOH}\) are soluble, \(\chem{Ba(OH)_2}\), \(\chem{Ca(OH)_2}\) are marginally soluble).
- Most \(\chem{S^{2-}}\), \(\chem{CO_3^{2-}}\), \(\chem{CrO_4^{2-}}\), \(\chem{PO_4^{3-}}\) salts are only slightly soluble, except for those containing the cations in Rule 2.
Describing Reactions in Solution
Formula Equation (Molecular Equation)
- Gives the overall reaction stoichiometry but not necessarily the actual forms of the reactants and products in solution.
- Reactants and products generally shown as compounds.
- Use solubility rules to determine which compounds are aqueous and which compounds are solids.
$$ \chem{AgNO_3(aq)+NaCl(aq)\rightarrow AgCl(s)+NaNO_3(aq)} $$
Complete Ionic Equation
- All substances that are strong electrolytes are represented as ions.
$$ \begin{align} \chem{Ag^+(aq)+NO_3^-(aq)+} & \chem{Na^+(aq)+Cl^-(aq)\rightarrow} \\ & \chem{AgCl(s)+Na^+(aq)+NO_3^-(aq)} \end{align} $$
- Includes only those solution components undergoing a change.
- Show only components that actually react.
- Spectator ions are not included (ions that do not participate directly in the reaction).
- \(\chem{Na^+}\) and \(\chem{NO_3^-}\) are spectator ions.
Stoichiometry of Precipitation Reactions
Solving Stoichiometry Problems for Reactions in Solution
- Identify the species present in the combined solution, and determine what reaction occurs.
- Write the balanced net ionic equation for the reaction.
- Calculate the moles of reactants.
- Determine which reactant is limiting.
- Calculate the moles of product(s), as required.
- Convert to grams or other units, as required.
Concept Check 1
10.0 mL of a 0.30 M sodium phosphate solution reacts with 20.0 mL of a 0.20 M lead(II) nitrate solution (assume no volume change).
- What precipitate will form $$ \phantom{ } $$
- What mass of precipitate will form? $$ \phantom{ } $$
Concept Check 1 - Answer
10.0 mL of a 0.30 M sodium phosphate solution reacts with 20.0 mL of a 0.20 M lead(II) nitrate solution (assume no volume change).
- What precipitate will form $$ \text{lead(II) phosphate, } \chem{Pb_3\left(PO_4\right)_2} $$
- What mass of precipitate will form? $$ 1.1\,\chem{g}\,\chem{Pb_3\left(PO_4\right)_2} $$
Acid-Base Reactions
Acid-Base Reactions (Brønsted-Lowry)
- Acid—proton donor
- Base—proton acceptor
- For a strong acid and base reaction: $$ \chem{H^+(aq)+OH^-(aq)\rightarrow H_2O(l)} $$
Performing Calculations for Acid-Base Reactions
- List the species present in the combined solution before any reaction occurs, and decide what reaction will occur.
- Write the balanced net ionic equation for this reaction.
- Calculate moles of reactants.
- Determine the limiting reactant, where appropriate.
- Calculate the moles of the required reactant or product.
- Convert to grams or volume (of solution), as required.
Acid-Base Titrations
- Titration – delivery of a measured volume of a solution of known concentration (the titrant) into a solution containing the substance being analyzed (the analyte).
- Equivalence point – enough titrant added to react exactly with the analyte.
- Endpoint – the indicator changes color so you can tell the equivalence point has been reached.
Concept Check 2
For the titration of sulfuric acid (\(\chem{H_2SO_4}\)) with sodium hydroxide (\(\chem{NaOH}\)), how many moles of sodium hydroxide would be required to react with 1.00 L of 0.500 M sulfuric acid to reach the endpoint? $$ \phantom{ } $$
Concept Check 2 - Answer
For the titration of sulfuric acid (\(\chem{H_2SO_4}\)) with sodium hydroxide (\(\chem{NaOH}\)), how many moles of sodium hydroxide would be required to react with 1.00 L of 0.500 M sulfuric acid to reach the endpoint? $$ 1.00\,\chem{mol}\,\chem{NaOH} $$
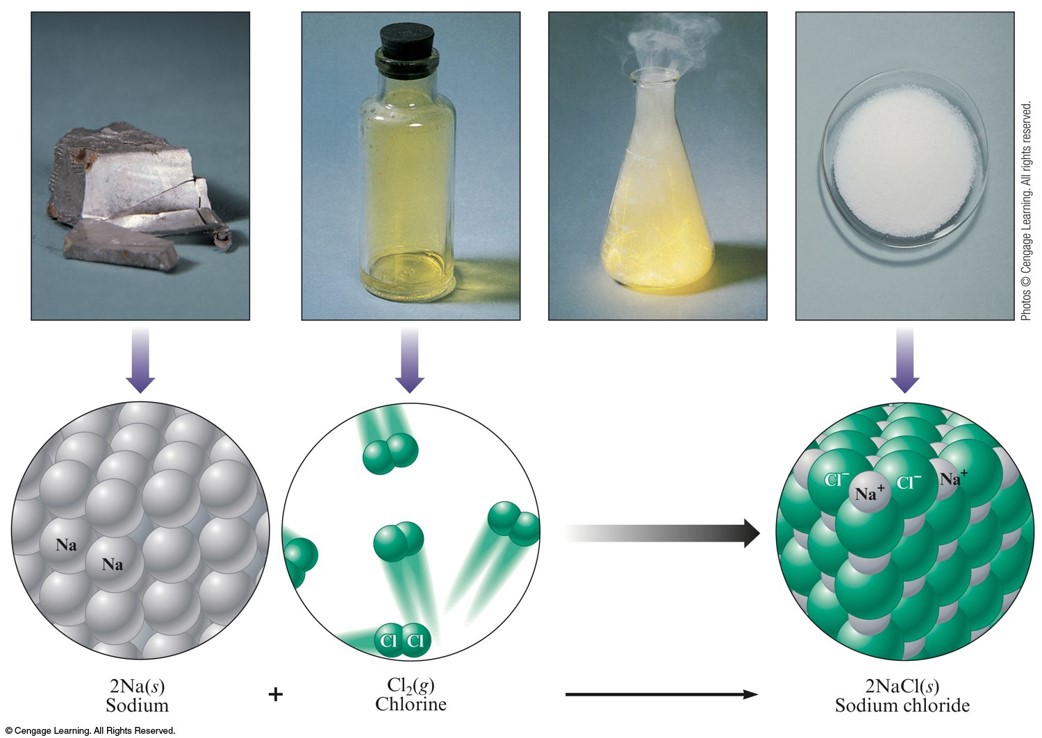
Oxidation-Reduction Reactions
Redox Reactions
- Reactions in which one or more electrons are transferred.
Reaction of Sodium and Chlorine
Rule for Assigning Oxidation States
- Oxidation state of an atom in an element = 0
- Oxidation state of monatomic ion = charge of the ion
- Oxygen = -2 in covalent compounds (except in peroxides where it = -1)
- Hydrogen = +1 in covalent compounds
- Fluorine = -1 in compounds
- Sum of oxidation states = 0 in compounds
- Sum of oxidation states = charge of the ion in ions
Exercise 1
Find the oxidation states for each of the elements in each of the following compounds:
- \(\chem{K_2Cr_2O_7}\)
- \(\chem{CO_3^{2-}}\)
- \(\chem{MnO_2}\)
- \(\chem{PCl_5}\)
- \(\chem{SF_4}\)
Exercise 1 - Answers
Find the oxidation states for each of the elements in each of the following compounds:
- \(\chem{K_2Cr_2O_7}\) \(\Rightarrow \chem{K=+1;\, Cr=+6;\, O=-2}\)
- \(\chem{CO_3^{2-}}\) \(\Rightarrow \chem{C=+4;\, O=-2}\)
- \(\chem{MnO_2}\) \(\Rightarrow \chem{Mn=+4;\, O=-2}\)
- \(\chem{PCl_5}\) \(\Rightarrow \chem{P=+5;\, Cl=-1}\)
- \(\chem{SF_4}\) \(\Rightarrow \chem{S=+4;\, F=-1}\)
Redox Characteristics
- Transfer of electrons
- Transfer may occur to form ions
- Oxidation – increase in oxidation state (loss of electrons); reducing agent
- Reduction – decrease in oxidation state (gain of electrons); oxidizing agent
Balancing Oxidation-Reduction Equations
Balancing Oxidation-Reduction Reactions by Oxidation States
- Write the unbalanced equation.
- Determine the oxidation states of all atoms in the reactants and products.
- Show electrons gained and lost using "tie lines."
- Use coefficients to equalize the electrons gained and lost.
- Balance the rest of the equation by inspection.
- Add appropriate states.
Example 1
Balance the reaction between solid zinc and aqueous hydrochloric acid to produce aqueous zinc(II) chloride and hydrogen gas.
- What is the unbalanced equation? $$ \chem{Zn(s)+HCl(aq)\rightarrow Zn^{2+}(aq)+Cl^-(aq)+H_2(g)} $$
- What are the oxidation states for each atom? $$ \chem{\underbrace{Zn}_0(s)+\underbrace{H}_{+1}\underbrace{Cl}_{-1}(aq)\rightarrow \underbrace{Zn^{2+}}_{+2}(aq)+\underbrace{Cl^-}_{-1}(aq)+\underbrace{H_2}_0(g)} $$
- How are electrons gained and lost? $$ \rlap{\overbrace{\phantom{\chem{Zn(s)+HCl(aq)\rightarrow Zn}}}^\text{1 electron gained (each atom)}}\chem{\underbrace{Zn}_0(s)}+\underbrace{\chem{\underbrace{H}_{+1}\underbrace{Cl}_{-1}(aq)}\rightarrow \chem{\underbrace{Zn^{2+}}_{+2}(aq)}+\chem{\underbrace{Cl^-}_{-1}(aq)}+\chem{\underbrace{H_2}_0}}_\text{2 electrons lost} \chem{(g)} $$ The oxidation state of chlorine remains unchanged.
Example 1 - cont.
- What coefficients are needed to equalize the electrons gained and lost? $$ \rlap{\overbrace{\phantom{\chem{Zn(s)+HCl(aq)\rightarrow Zn}}}^\text{1 electron gained (each atom) $\times$ 2}}\chem{\underbrace{Zn}_0(s)}+\underbrace{\chem{\underbrace{H}_{+1}\underbrace{Cl}_{-1}(aq)}\rightarrow \chem{\underbrace{Zn^{2+}}_{+2}(aq)}+\chem{\underbrace{Cl^-}_{-1}(aq)}+\chem{\underbrace{H_2}_0}}_\text{2 electrons lost} \chem{(g)} $$ $$ \chem{Zn(s) + 2HCl(aq) \rightarrow Zn^{2+}(aq)+Cl^-(aq)+H_2(g)} $$
- What coefficients are needed to balance the remaining elements? $$ \chem{Zn(s) + 2HCl(aq) \rightarrow Zn^{2+}(aq)+2Cl^-(aq)+H_2(g)} $$
/