Chapter 19
Transition Metals and Coordination Chemistry
Shaun Williams, PhD
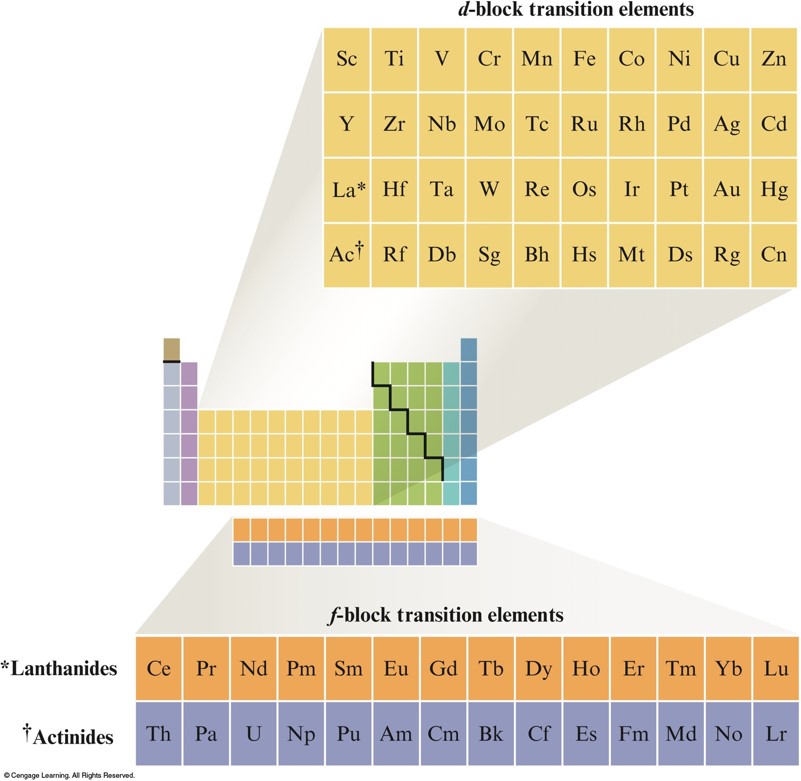
Transition Metals
- Show great similarities within a given period as well as within a given vertical group.
The Postion of the Transition Elements on the Periodic Table
Forming Ionic Compounds
- More than one oxidation state is often found.
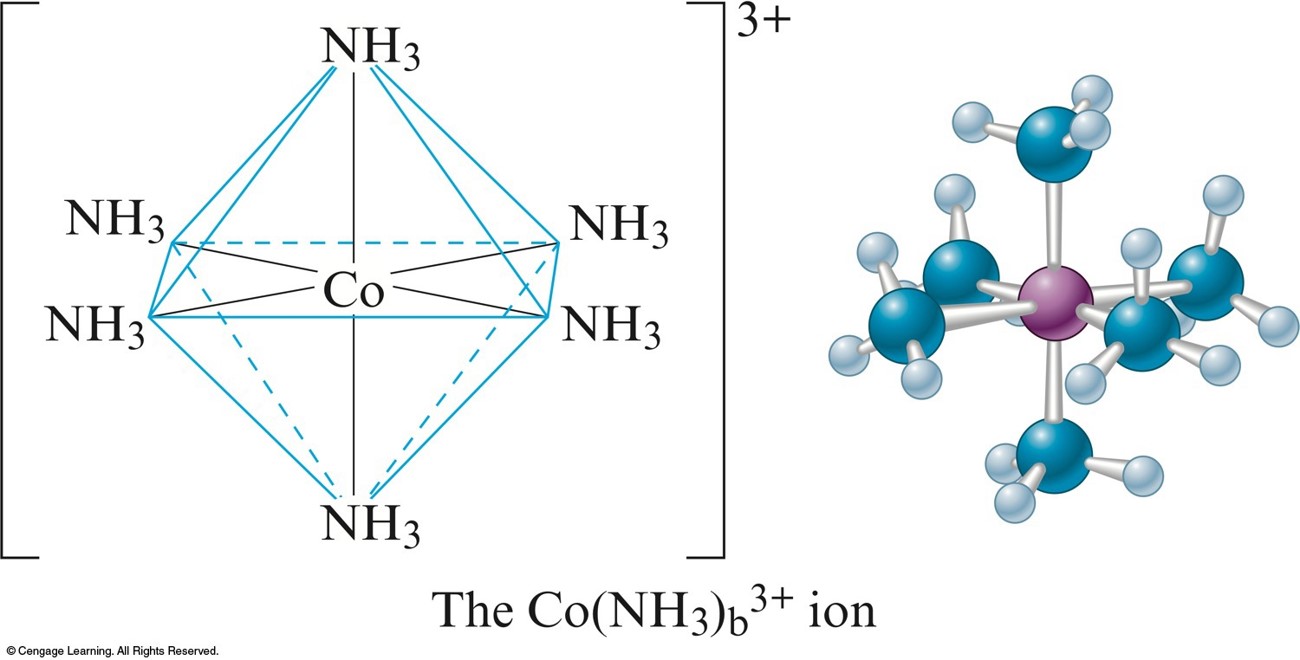
- Cations are often complex ions – species where the transition metal ion is surrounded by a certain number of ligands (Lewis bases).
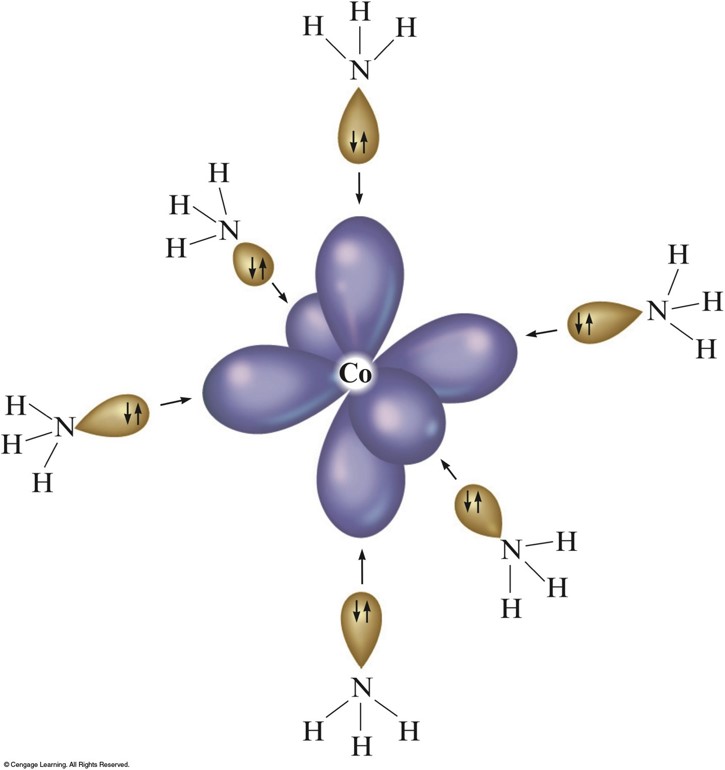
The Complex Ion \(\chem{Co(NH_3)_6^{3+}}\)
Ionic Compounds with Transition Metals
- Most compounds are colored because the transition metal ion in the complex ion can absorb visible light of specific wavelengths.
- Many compounds are paramagnetic.
Electron Configurations
- Example
- \(\chem{V}\): \(\chem{[Ar]4s^23d^3}\)
- Exceptions: \(\chem{Cr}\) and \(\chem{Cu}\)
- \(\chem{Cr}\): \(\chem{[Ar]4s^13d^5}\)
- \(\chem{Cu}\): \(\chem{[Ar]4s^13d^{10}}\)
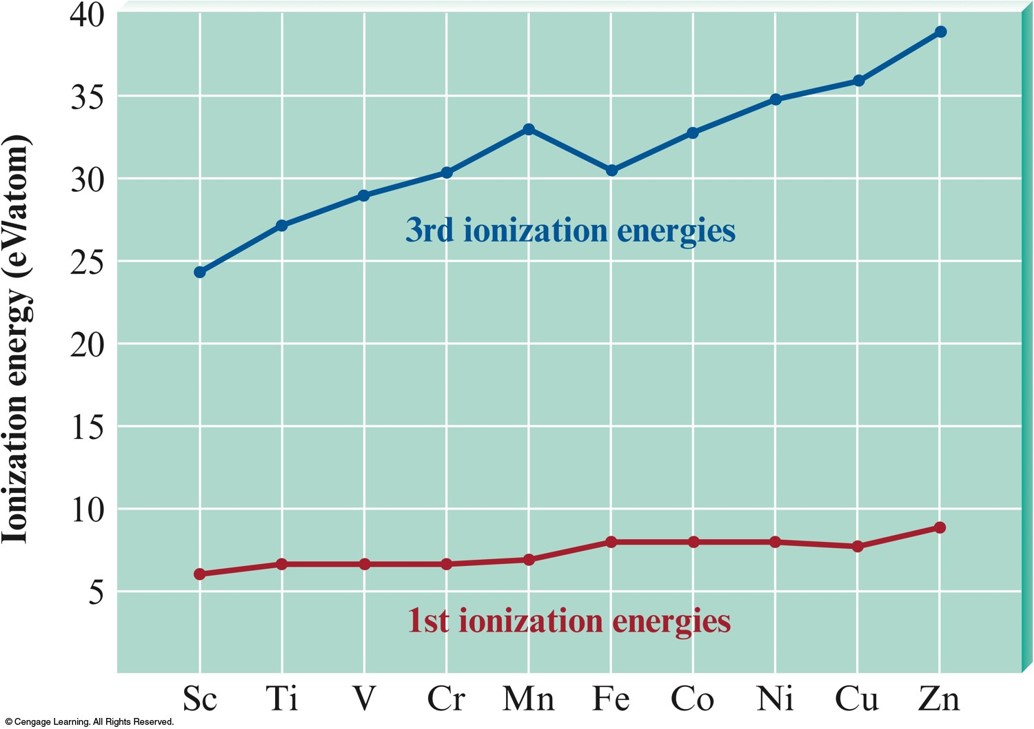
- First-row transition metal ions do not have 4s electrons.
- Energy of the 3d orbitals is significantly less than that of the 4s orbital.
Concept Check
What is the expected electron configuration of \(\chem{Sc^+}\)?
\(\chem{[Ar]3d^2}\)
Plots of the First (Red Dots) and Third (Red Dots) Ionization Energies for the First-Row Transition Metals
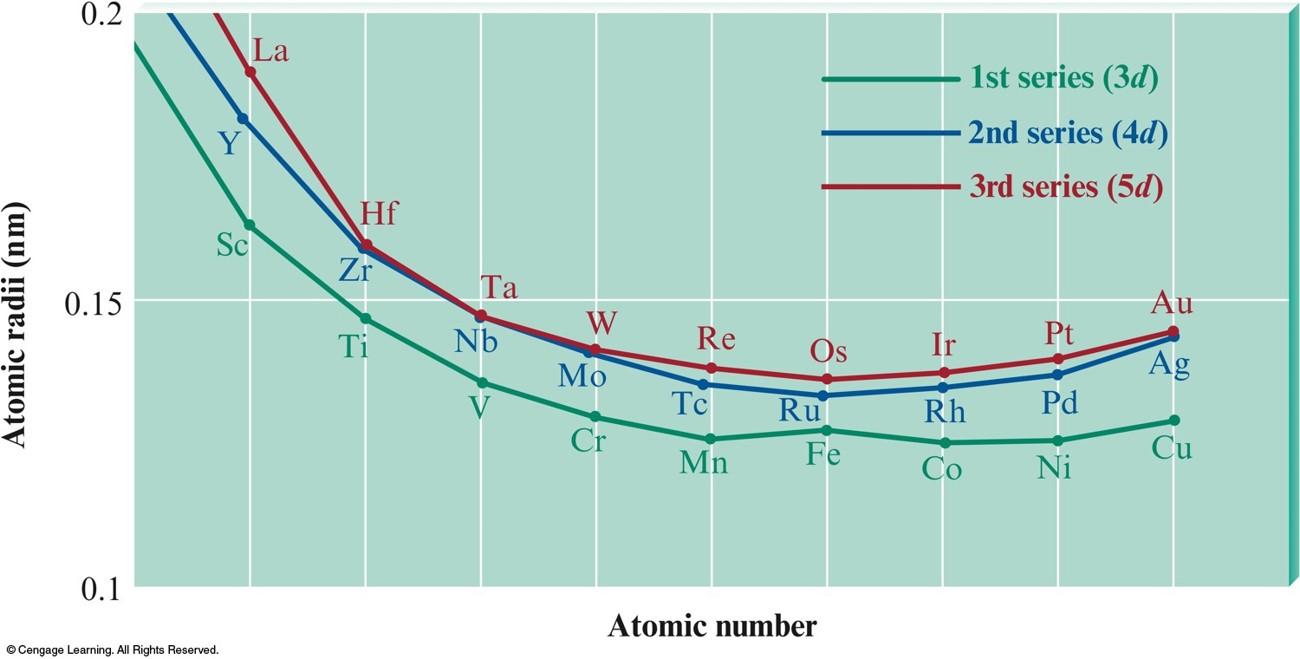
Atomic Radii of the \(3d\), \(4d\), and \(5d\) Transition Series
The First-Row Transition Metals
- 3d transition metals
- Scandium – chemistry strongly resembles lanthanides
- Titanium – excellent structural material (light weight)
- Vanadium – mostly in alloys with other metals
- Chromium – important industrial material
- Manganese – production of hard steel
- Iron – most abundant heavy metal
- Cobalt – alloys with other metals
- Nickel – plating more active metals; alloys
- Copper – plumbing and electrical applications
- Zinc – galvanizing steel
Oxidation States and Species for Vanadium in Aqueous Solution
| Oxidation Stae of Vanadium | Species in Aqueous Solution |
|---|---|
| +5 | \(\chem{VO_2^+}\) (yellow) |
| +4 | \(\chem{VO^{2+}}\) (blue) |
| +3 | \(\chem{V^{3+}(aq)}\) (blue-green) |
| +2 | \(\chem{V^{2+}(aq)}\) (violet) |
Typical Chromium Compounds
| Oxidation State of Chromium | Examples of Compound (\(\chem{X}\) is a halogen) |
|---|---|
| +2 | \(\chem{CrX_2}\) |
| +3 | \(\chem{CrX_3}\) (green) \(\chem{Cr_2O_3}\) (blue-green) |
| +6 | \(\chem{K_2Cr_2O_7}\) (orange) \(\chem{Na_2CrO_4}\) (yellow) \(\chem{CrO_3}\) (red) |
Some Compounds of Manganese in Its Most Common Oxidation States
| Oxidation State of Manganese | Examples of Compound |
|---|---|
| +2 | \(\chem{Mn(OH)_2}\) (pink) \(\chem{MnS}\) (salmon) \(\chem{MnSO_4}\) (reddish) \(\chem{MnCl_2}\) (pink) |
| +4 | \(\chem{MnO_4}\) (dark brown) |
| +7 | \(\chem{KMnO_4}\) (purple) |
Typical Iron Compounds
| Oxidation State of Iron | Examples of Compounds |
|---|---|
| +2 | \(\chem{FeO}\) (black) \(\chem{FeS}\) (brownish black) \(\chem{FeSO_4\cdot 7H_2O}\) (green) \(\chem{K_4Fe(CN)_6}\) (yellow) |
| +3 | \(\chem{FeCl_3}\) (brownish black) \(\chem{Fe_2O_3}\) (reddish brown) \(\chem{K_3Fe(CN)_6}\) (red) \(\chem{Fe(SCN)_3}\) (red) |
| +2, +3 (mixture) | \(\chem{Fe_3O_4}\) (black) \(\chem{KFe[Fe(CN)_6]}\) (deep blue, "Prussian blue") |
Typical Cobalt Compounds
| Oxidation State of Cobalt | Examples of Compounds |
|---|---|
| +2 | \(\chem{CoSO_4}\) (dark blue) \(\chem{[Co(H_2O)_6]Cl_2}\) (pink) \(\chem{[Co(H_2O)_6](NO_3)_2}\) (red) \(\chem{CoS}\) (black) \(\chem{CoO}\) (greenish brown) |
| +3 | \(\chem{CoF_3}\) (brown) \(\chem{Co_2O_3}\) (charcoal) \(\chem{K_3[Co(CN)_6]}\) (yellow) \(\chem{[Co(NH_3)_6]Cl_3}\) (yellow) |
Typical Nickel Compounds
| Oxidation State of Nickel | Examples of Compounds |
|---|---|
| +2 | \(\chem{NiCl_2}\) (yellow) \(\chem{[Ni(H_2O)_6]Cl_2}\) (green) \(\chem{NiO}\) (greenish black) \(\chem{NiS}\) (black) \(\chem{[Ni(H_2O)_6]SO_4}\) (green) \(\chem{[Ni(NH_3)_6](NO_3)_2}\) (blue) |
Typical Copper Compounds
| Oxidation State of Copper | Examples of Compounds |
|---|---|
| +1 | \(\chem{Cu_2O}\) (red) \(\chem{Cu_2S}\) (black) \(\chem{CuCl}\) (white) |
| +2 | \(\chem{CuO}\) (black) \(\chem{CuSO_4\cdot 5H_2O}\) (blue) \(\chem{CuCl_2\cdot 2H_2O}\) (green) \(\chem{[Cu(H_2O)_6](NO_3)_2}\) (blue) |
Alloys Containing Copper
| Alloy | Composition (% by mass) |
|---|---|
| Brass | \(\chem{Cu}\) (20-97), \(\chem{Zn}\) (2-80), \(\chem{Sn}\) (0-14), \(\chem{Pb}\) (0-12), \(\chem{Mn}\) (0-25) |
| Bronze | \(\chem{Cu}\) (50-98), \(\chem{Sn}\) (0-35), \(\chem{Zn}\) (0-29), \(\chem{Pb}\) (0-50), \(\chem{P}\) (0-3) |
| Sterling silver | \(\chem{Cu}\) (7.5), \(\chem{Ag}\) (92.5) |
| Gold (18-karat) | \(\chem{Cu}\) (5-15), \(\chem{Au}\) (75), \(\chem{Ag}\) (10-20) |
| Gold (14-karat) | \(\chem{Cu}\) (12-28), \(\chem{Au}\) (58), \(\chem{Ag}\) (4-30) |
A Coordination Compound
- Typically consists of a complex ion and counterions (anions or cations as needed to produce a neutral compound): $$ \begin{align} &\chem{[Co(NH_3)_5Cl]Cl_2} \\ &\chem{[Fe(en)_2(NO_2)_2]_2SO_4} \\ &\chem{K_3Fe(CN)_6} \end{align} $$
- Coordination Number
- Number of bonds formed between the metal ion and the ligands in the complex ion.
- 6 and 4 (most common)
- 2 and 8 (least common)
Ligands
- Neutral molecule or ion having a lone electron pair that can be used to form a bond to a metal ion.
- Monodentate ligand – one bond to a metal ion,
- Bidentate ligand (chelate) – two bonds to a metal ion,
- Polydentate ligand – more than two bonds to a metal ion
- Coordinate Covalent Bond
- Bond resulting from the interaction between a Lewis base (the ligand) and a Lewis acid (the metal ion).
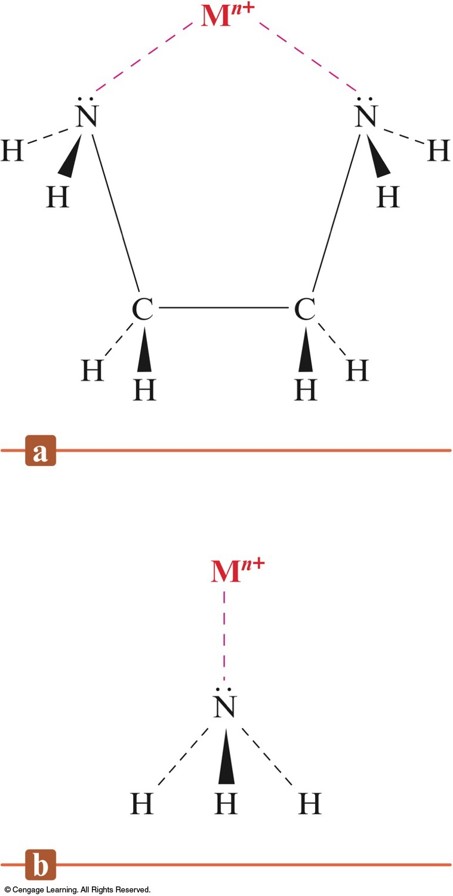
The Bidentate Ligand Ethylenediamine and the Monodentate Ligand Ammonia
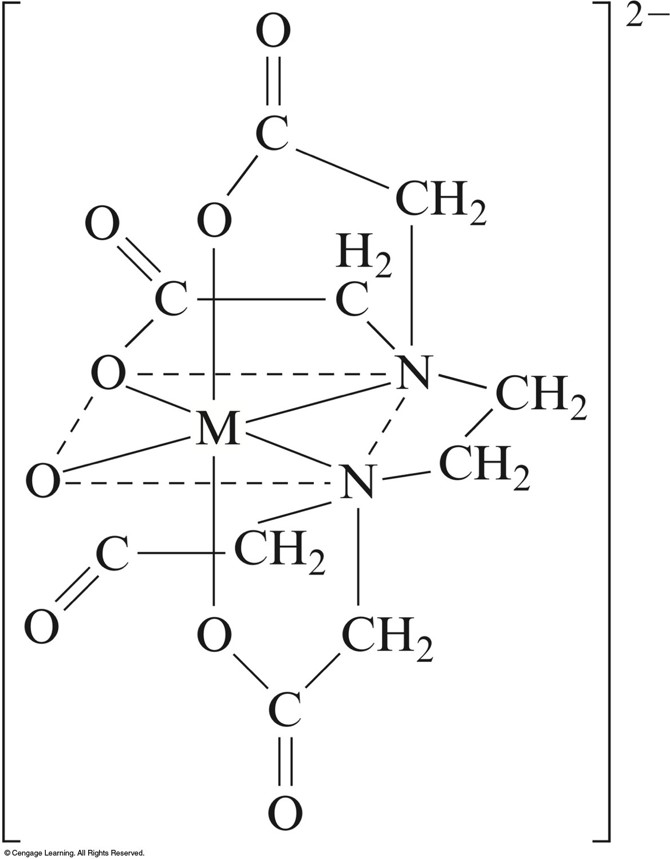
The Coordination of EDTA with a 2+ Metal Ions
ethylenediaminetetraacetate
Rules for Naming Coordination Compounds
$$ \chem{[Co(NH_3)_5Cl]Cl_2} $$
- Cation is named before the anion.
- "chloride" goes last (the counterion)
- Ligands are named before the metal ion.
- ammonia (ammine) and chlorine
- (chloro) named before cobalt
- For negatively charged ligands, an "o" is added to the root name of an anion (such as fluoro, bromo, chloro, etc.).
- The prefixes mono-, di-, tri-, etc., are used to denote the number of simple ligands.
- penta ammine
Rules for Naming Coordination Compounds (cont.)
$$ \chem{[Co(NH_3)_5Cl]Cl_2} $$
- The oxidation state of the central metal ion is designated by a Roman numeral:
- cobalt (III)
- When more than one type of ligand is present, they are named alphabetically:
- pentaamminechloro
- If the complex ion has a negative charge, the suffix "ate" is added to the name of the metal.
- The correct name is:
pentaamminechlorocobalt(III) chloride
Exercise
Name the following coordination compounds.
- \(\chem{[Co(H_2O)_6]Br_3}\)
- \(\chem{Na_2[PtCl_4]}\)
hexaaquacobalt(III) bromide
sodiumtetrachloro-platinate(II)
Some Classes of Isomers
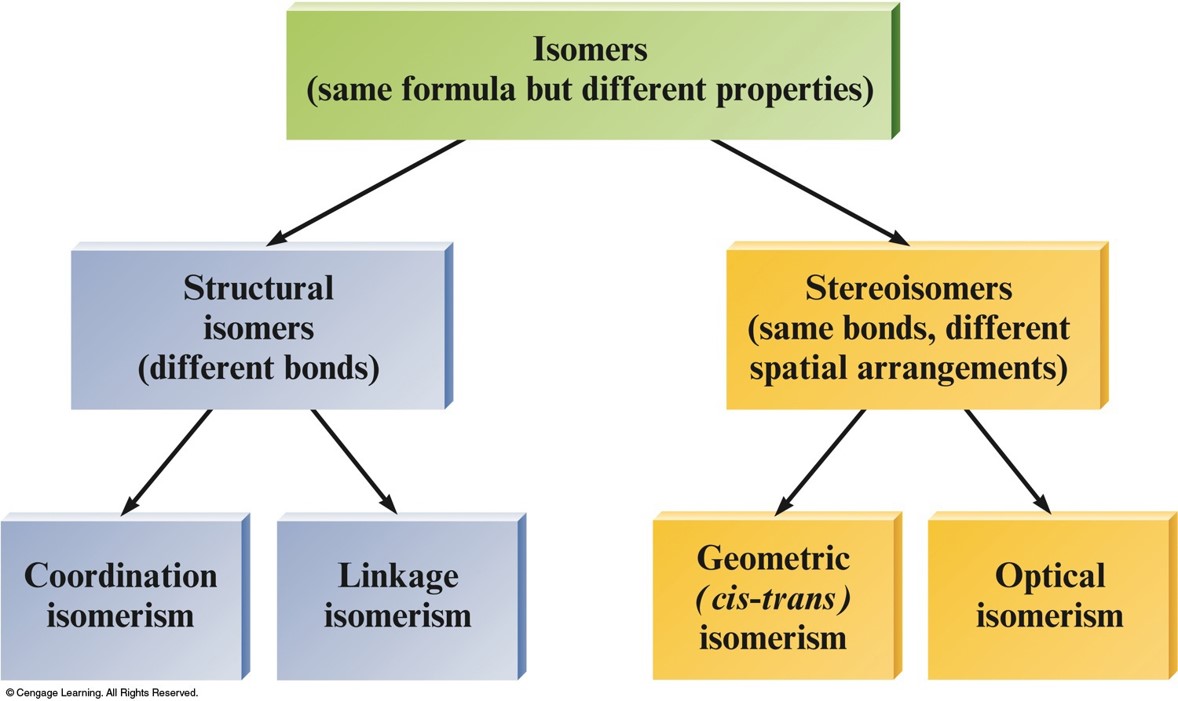
Structural Isomerism
- Coordination Isomerism:
- Composition of the complex ion varies.
- \(\chem{[Cr(NH_3)_5SO_4]Br}\) and \(\chem{[Cr(NH_3)_5Br]SO_4}\)
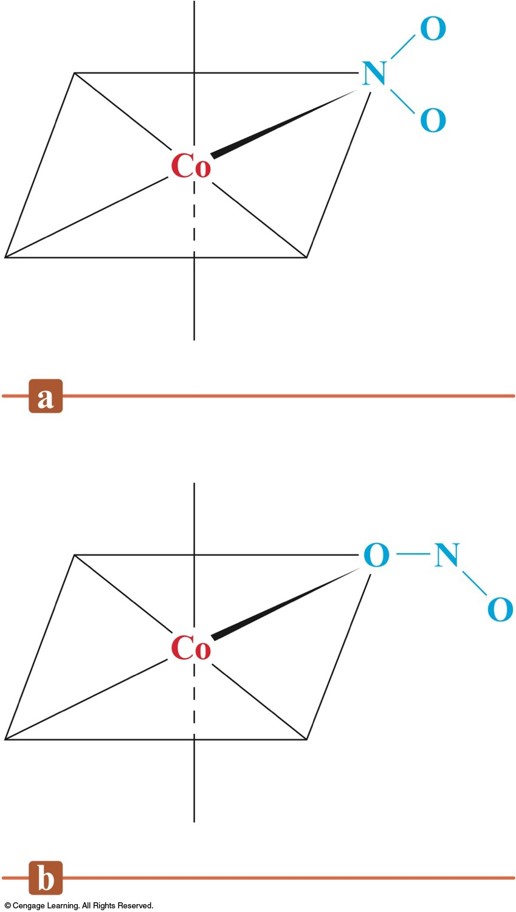
- Linkage Isomerism:
- Composition of the complex ion is the same, but the point of attachment of at least one of the ligands differs.
Linkage Isomerism of \(\chem{NO_2^-}\)
Stereoisomerism
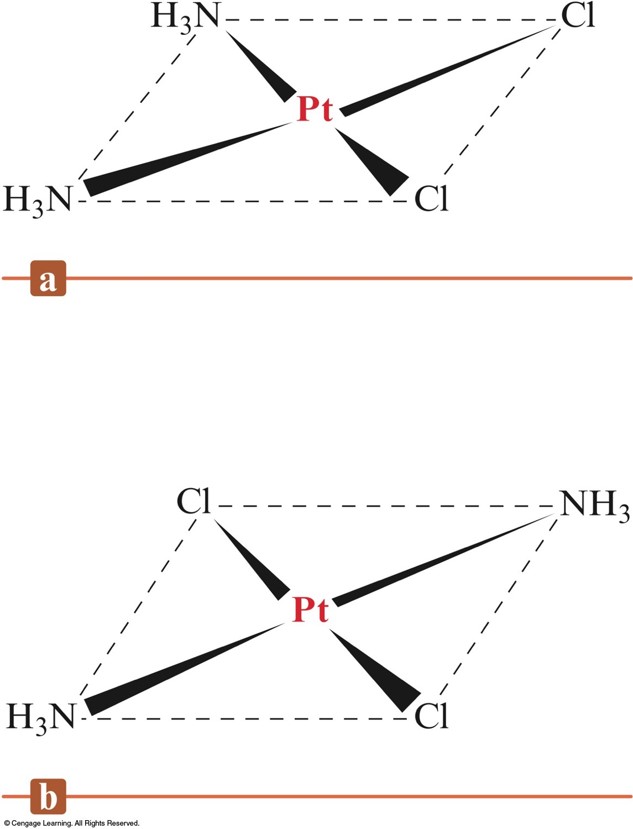
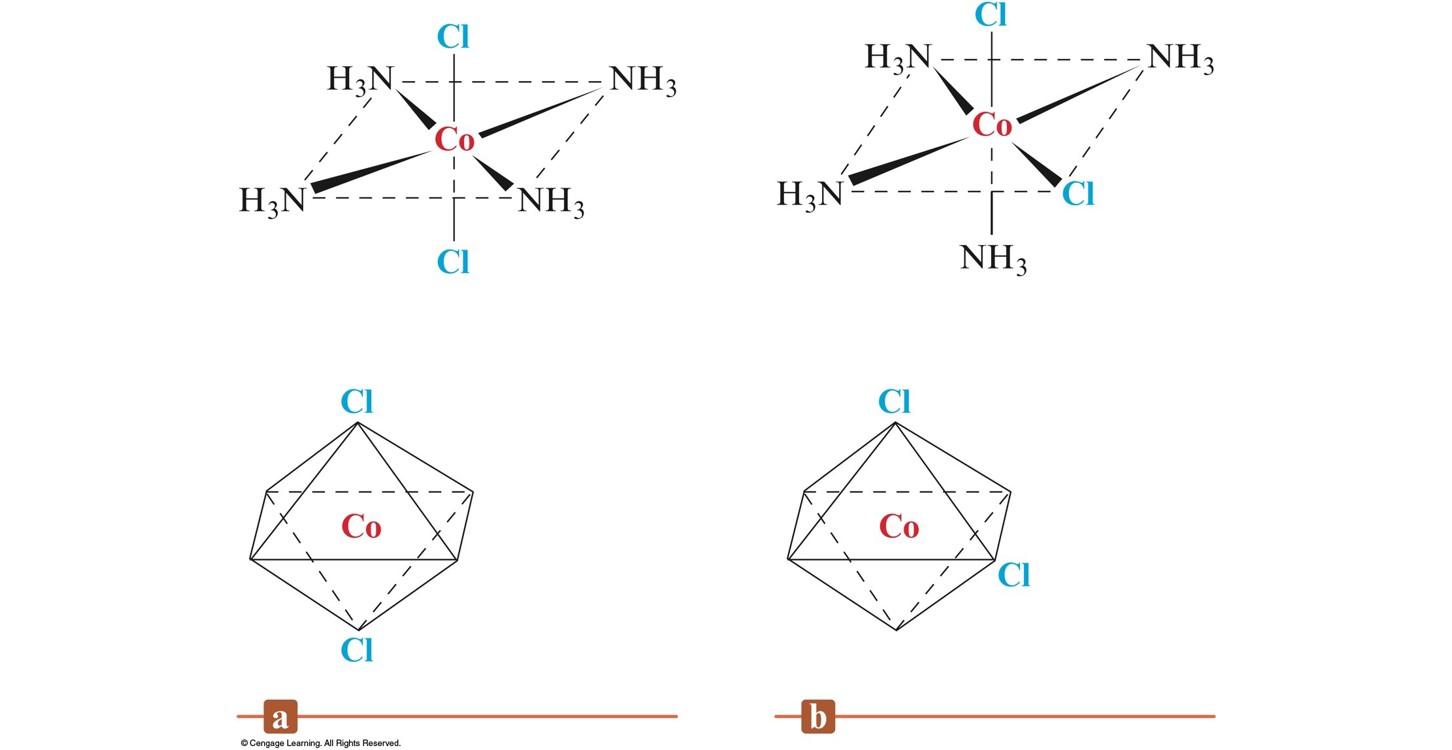
- Geometrical Isomerism (cis-trans):
- Atoms or groups of atoms can assume different positions around a rigid ring or bond.
- cis – same side (next to each other)
- trans – opposite sides (across from each other)
Geometrical (cis-trans) Isomerism for a Square Planar Compound
Geometrical (cis-trans) Isomerism for an Octahedral Complex Ion
Steroisomerism
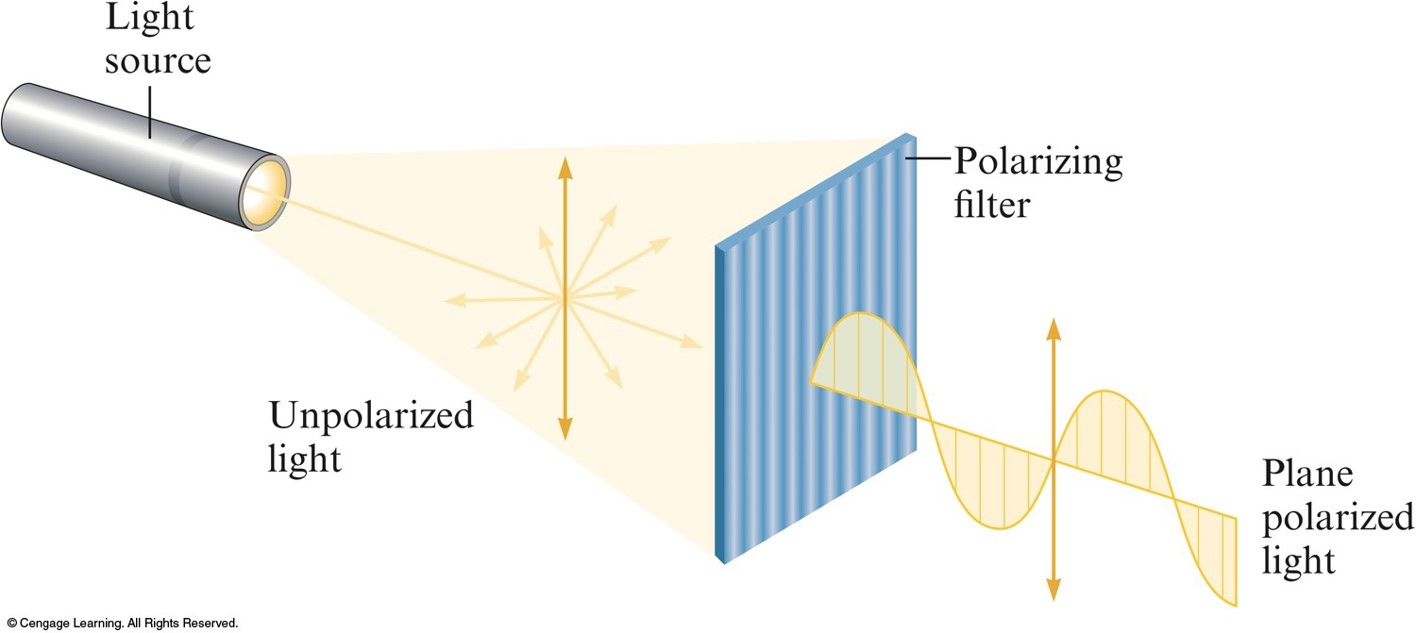
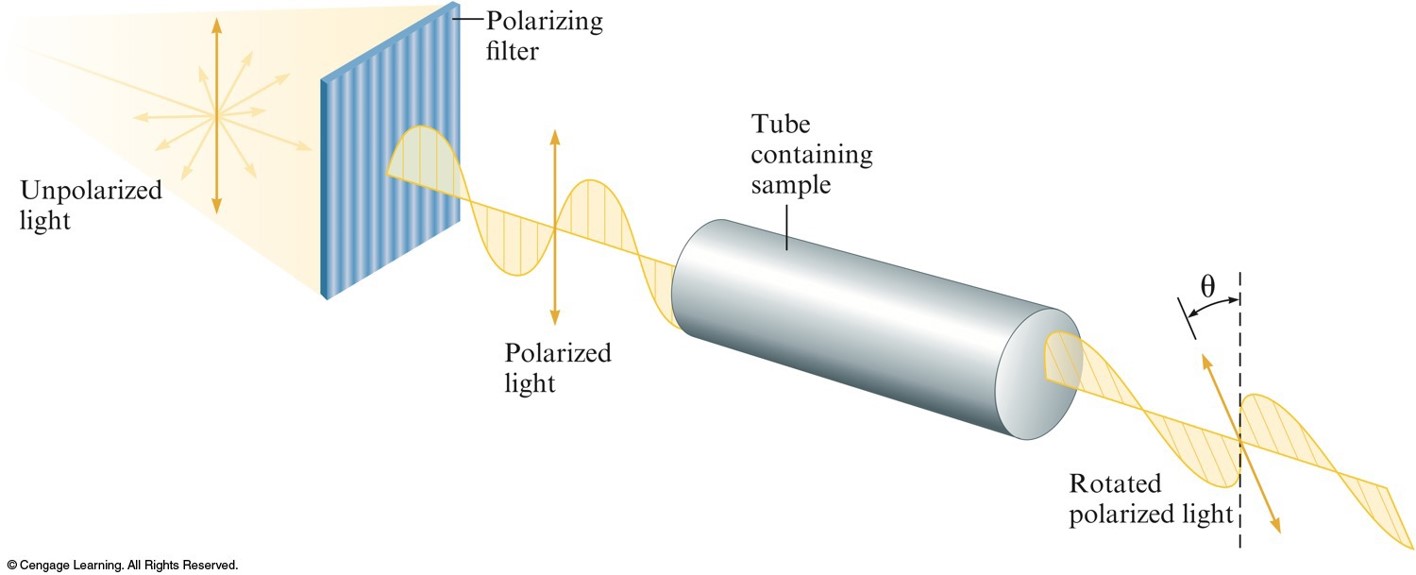
- Optical Isomerism:
- Isomers have opposite effects on plane-polarized light.
Unpolarized Light Consists of Waves Vibrating in Many Different Planes
The Rotation of the Plane of Polarized Light by an Optically Active Substance
Optical Activity
- Exhibited by molecules that have nonsuperimposable mirror images (chiral molecules).
- Enantiomers – isomers of nonsuperimposable mirror images.
A Human Hand Exhibits a Nonsuperimposable Mirror Image

Concept Check
Does \(\chem{[Co(en)_2Cl_2]Cl}\) exhibit geometrical isomerism?
Yes
Does it exhibit optical isomerism?
trans form - no
cis form - yes
Bonding in Complex Ions
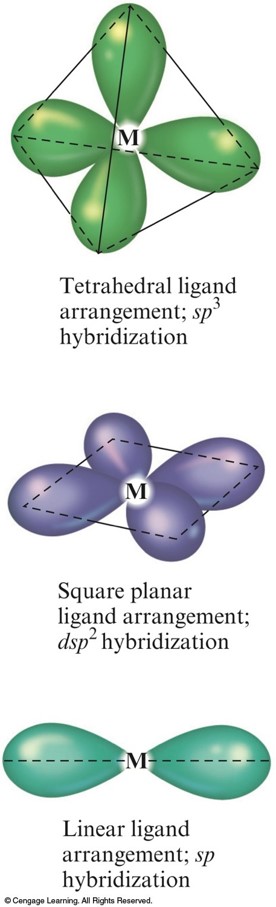
- The VSEPR model for predicting structure generally does not work for complex ions.
- However, assume a complex ion with a coordination number of 6 will have an octahedral arrangement of ligands.
- And, assume complexes with two ligands will be linear.
- But, complexes with a coordination number of 4 can be either tetrahedral or square planar.
- The interaction between a metal ion and a ligand can be viewed as a Lewis acid–base reaction with the ligand donating a lone pair of electrons to an empty orbital of the metal ion to form a coordinate covalent bond.
The Interaction Between a Metal Ion and a Ligand Can Be Viewed as a Lewis Acid-Base Reaction
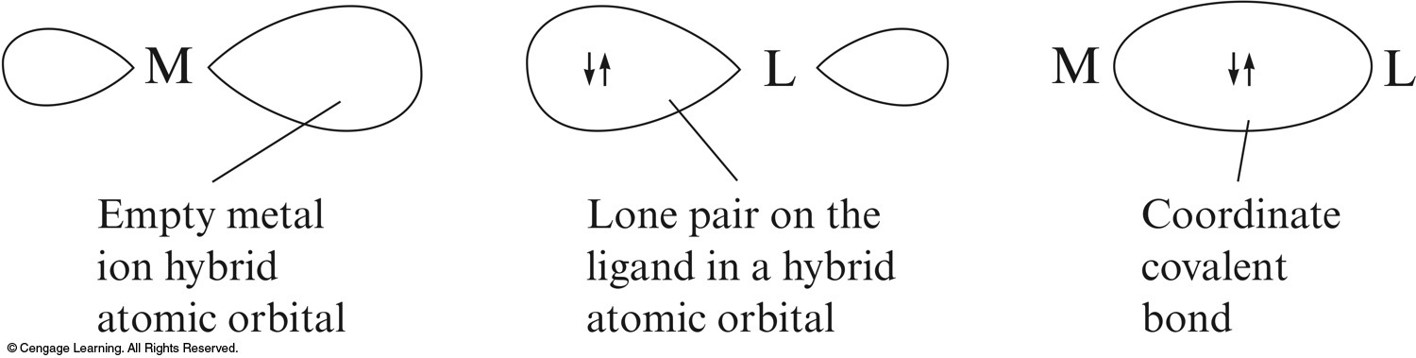
Hybrid Orbitals on \(\chem{Co^{3+}}\) Can Accept an Electron Pair from Each \(\chem{NH_3}\) Ligand
The Hybrid Orbitals Required for Tetrahedral, Square Planar, and Linear Complex Ions
The Crystal Field Model
- Focuses on the energies of the \(d\) orbitals.
Assumptions
- Ligands are negative point charges.
- Metal–ligand bonding is entirely ionic:
- strong-field (low–spin):
- weak-field (high–spin):
large splitting of \(d\) orbitals
small splitting of \(d\) orbitals
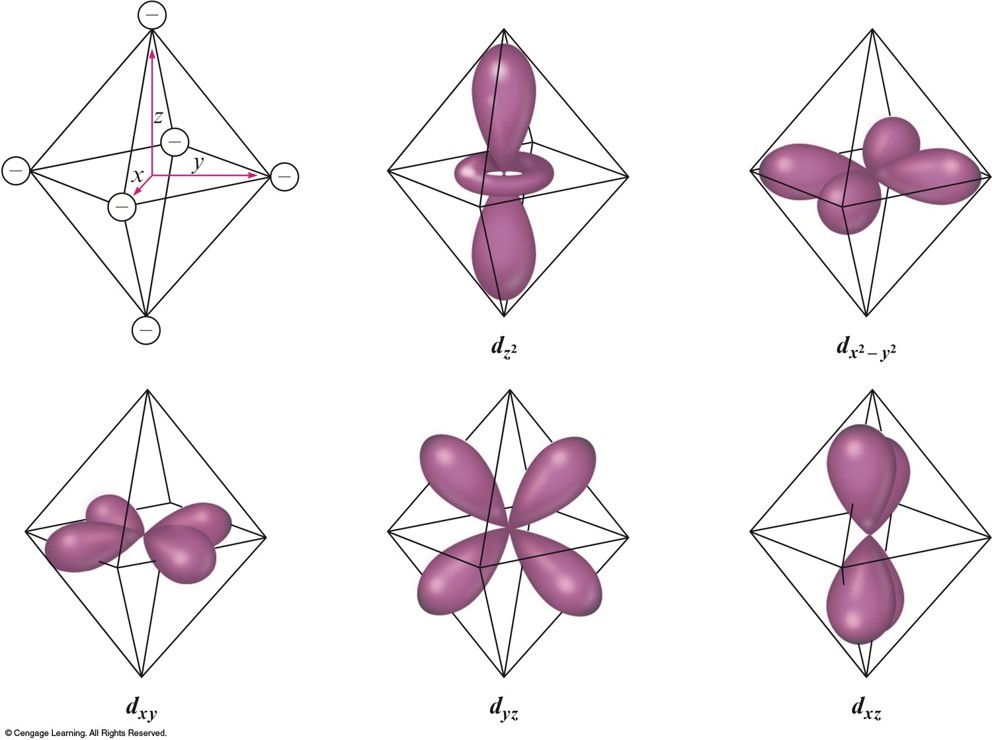
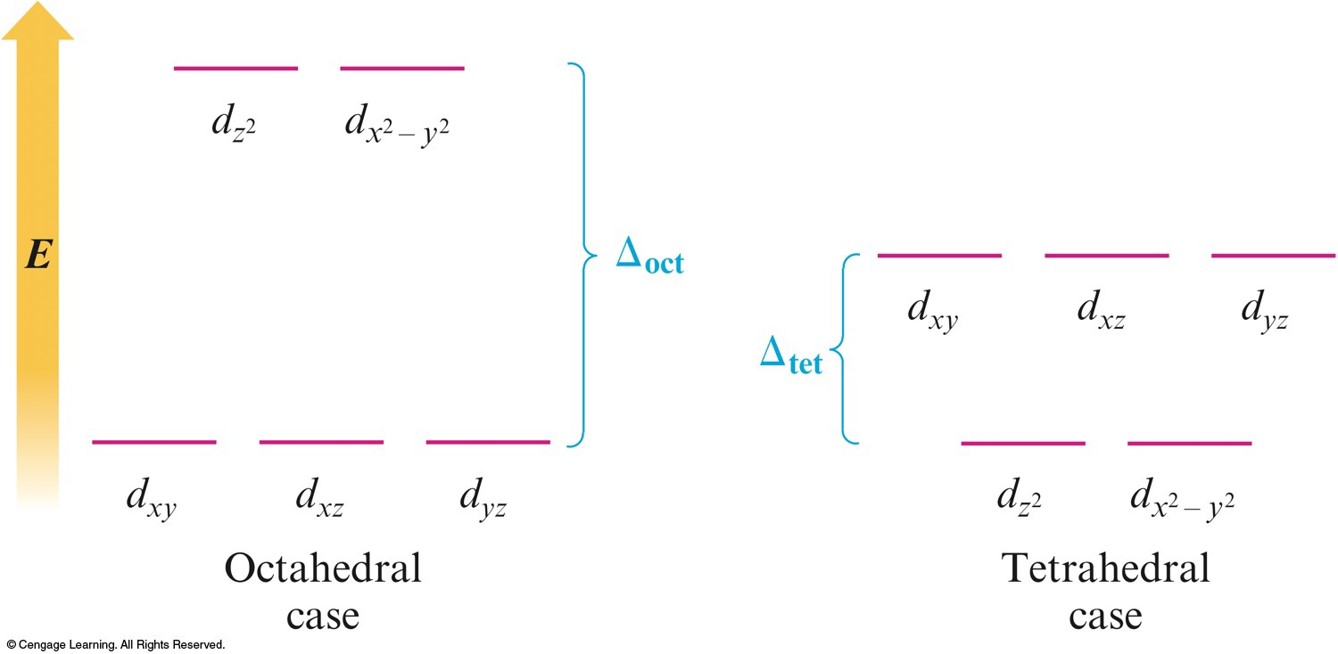
Octahedral Complexes
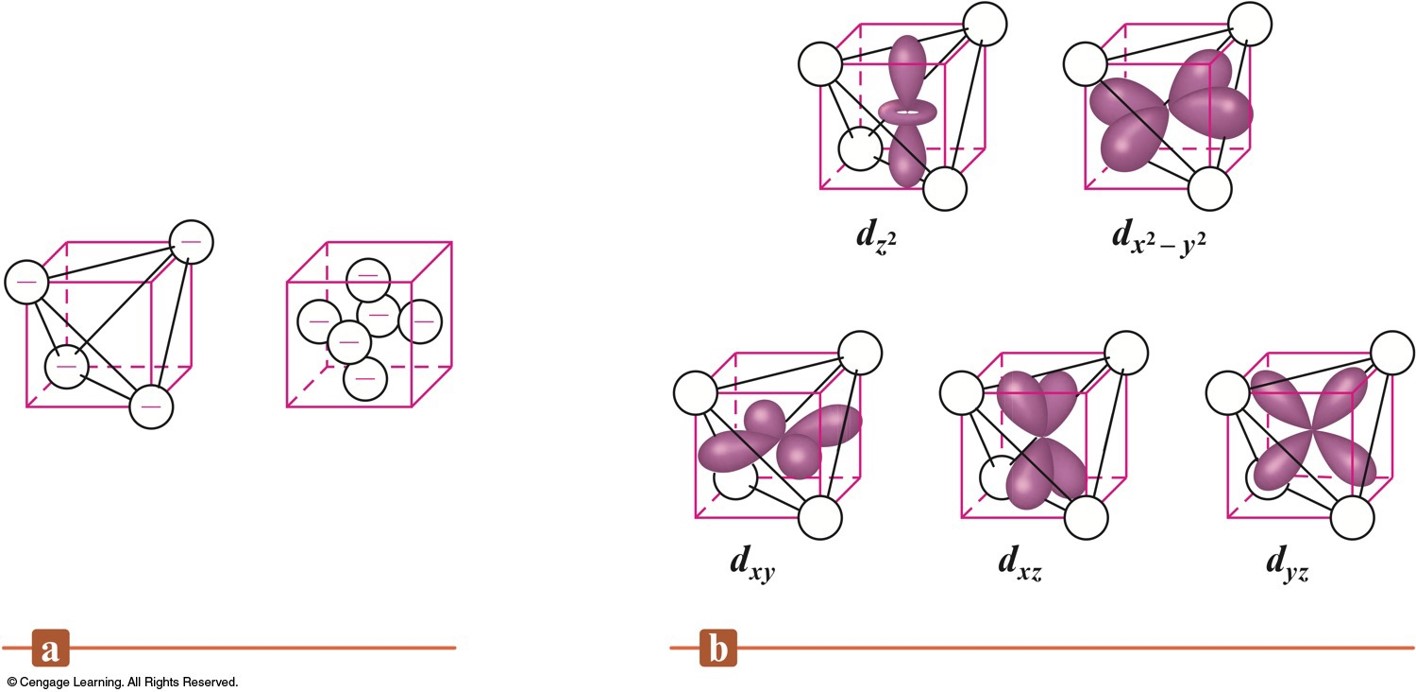
- \(d_{z^2}\) and \(d_{x^2-y^2}\) point their lobes directly at the point-charge ligands.
- \(d_{xz}\), \(d_{yz}\), and \(d_{xy}\) point their lobes between the point charges.
An Octahedral Arrangement of Point-Charge Ligands and the Orientation of the 3d Orbitals
Which Type of Orbital is Lower in Energy?
- Because the negative point-charge ligands repel negatively charged electrons, the electrons will first fill the \(d\) orbitals farthest from the ligands to minimize repulsions.
- The \(d_{xz}\), \(d_{yz}\), and \(d_{xy}\) orbitals are at a lower energy in the octahedral complex than are the \(d_{z^2}\) and \(d_{x^2-y^2}\) orbitals.
The Energies of the \(3d\) Orbitals for a Metal Ion in an Octahedral Complex
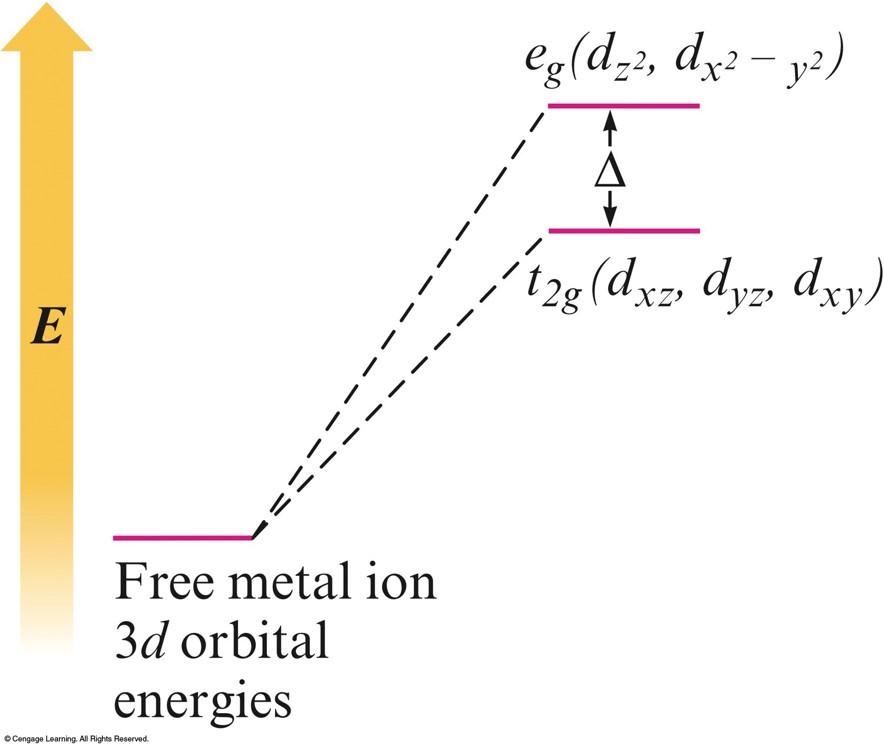
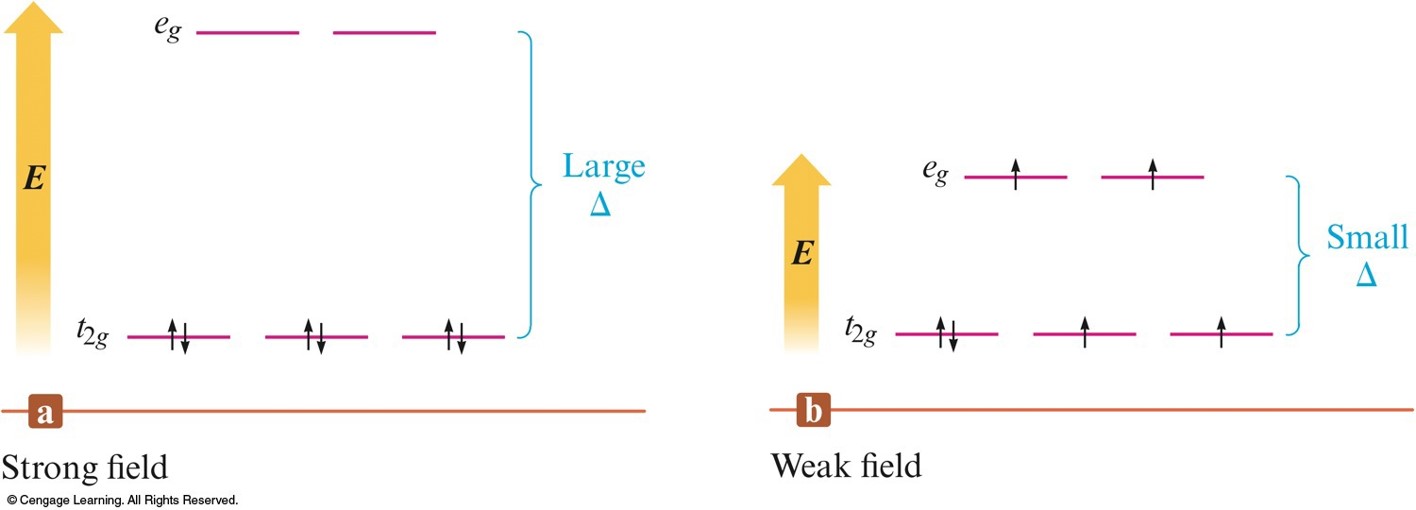
Possible Electron Arrangements in the Split \(3d\) Orbitals in an Octahedral Complex of \(\chem{Co^{3+}}\)
Magnetic Properties
- Strong–field (low–spin):
- Yields the minimum number of unpaired electrons.
- Weak–field (high–spin):
- Gives the maximum number of unpaired electrons.
- Hund's rule still applies.
Spectrochemical Series
- Strong–field ligands to weak–field ligands.
- Magnitude of split for a given ligand increases as the charge on the metal ion increases.
$$\begin{align} &\chem{CN^-} & \text{(large split)} \\ &\chem{NO_2^-} & \\ &\chem{en} & \\ &\chem{NH_3} & \\ &\chem{H_2O} & \\ &\chem{OH^-} & \\ &\chem{F^-} & \\ &\chem{Cl^-} & \\ &\chem{Br^-} & \\ &\chem{I^-} & \text{(small split)} \end{align}$$
Complex Ion Colors
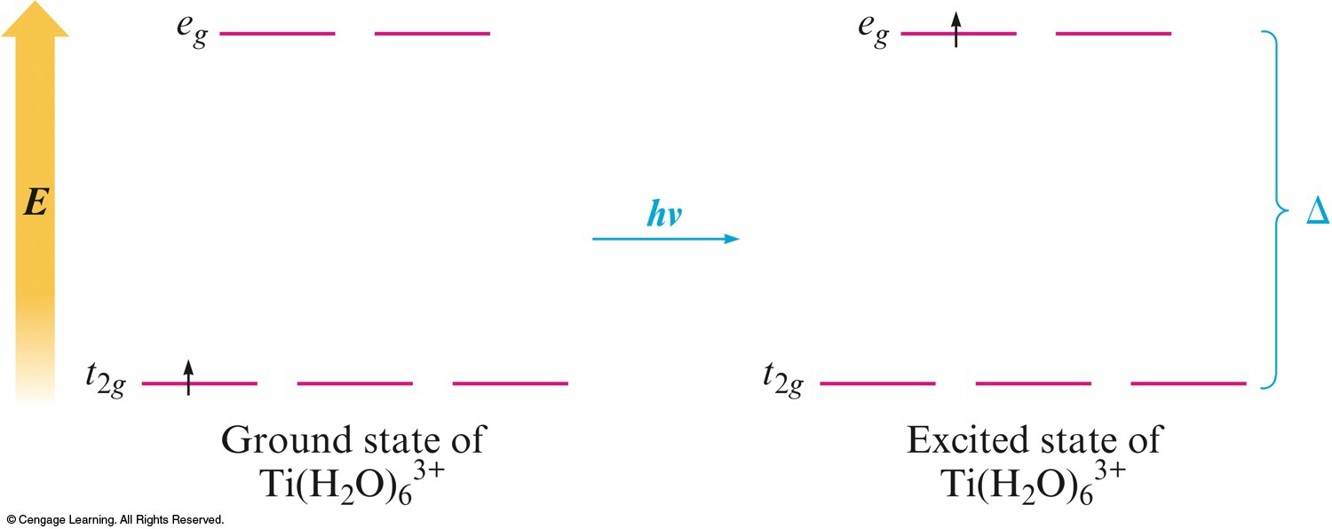
- When a substance absorbs certain wavelengths of light in the visible region, the color of the substance is determined by the wavelengths of visible light that remain.
- Substance exhibits the color complementary to those absorbed.
- The ligands coordinated to a given metal ion determine the size of the \(d\)–orbital splitting, thus the color changes as the ligands are changed.
- A change in splitting means a change in the wavelength of light needed to transfer electrons between the \(t_{2g}\) and \(e_g\) orbitals.
Absorbtion of Visible Light by the Complex Ion \(\chem{Ti(H_2O)_6^{3+}}\)
Concept Check
Which of the following are expected to form colorless octahedral compounds? \(\chem{Zn^{2+},\,Fe^{2+},\,Mn^{2+},\,Cu^{+},\,Cr^{3+},\,Ti^{4+},\,Ag^{+},\,Fe^{3+},\,Cu^{2+},\,Ni^{2+}}\)
\(\chem{Zn^{2+},\,Cu^{+},\,Ti^{4+},\,Ag^{+}}\)
Tetrahedral Arrangement
- None of the \(3d\) orbitals "point at the ligands".
- Difference in energy between the split \(d\) orbitals is significantly less.
- \(d\)–orbital splitting will be opposite to that for the octahedral arrangement.
- Weak–field case (high–spin) always applies.
The \(d\) Orbitals in a Tetrahedral Arrangement of Point Charges
The Crystal Field Diagrams for Octahedral and Tetrahedral Complexes
Concept Check
Consider the Crystal Field Model (CFM).
- Which is lower in energy, \(d\)–orbital lobes pointing toward ligands or between?
- The electrons in the \(d\)–orbitals – are they from the metal or the ligands?
- Why would electrons choose to pair up in \(d\)–orbitals instead of being in separate orbitals?
- Why is the predicted splitting in tetrahedral complexes smaller than in octahedral complexes?
between
metal
Since some orbitals are higher in energy than others (see "1"), electrons may actually be lower in energy by pairing up than by jumping up in energy to be in a separate orbital.
In an octahedral geometry there are some orbitals pointing directly at ligands. Thus, there is a greater energy difference between these (larger splitting).
Concept Check
Using the Crystal Field Model, sketch possible electron arrangements for the following. Label one sketch as strong field and one sketch as weak field.
- \(\chem{Ni(NH_3)_6^{2+}}\)
- \(\chem{Fe(CN)_6^{3-}}\)
- \(\chem{Co(NH_3)_6^{3+}}\)
A \(d^8\) ion will look the same as strong field or weak field in an octahedral complex. In each case there are two unpaired electrons.
This is a \(d^5\) ion. In the weak field case, all five electrons are unpaired. In the strong field case, there is one unpaired electron.
This is a \(d^6\) ion. In the weak field case, there are four unpaired electrons. In the strong field case, there are no unpaired electrons.
Concept Check
A metal ion in a high–spin octahedral complex has 2 more unpaired electrons than the same ion does in a low–spin octahedral complex.
What are some possible metal ions for which this would be true?
Metal ions would need to be \(d^4\) or \(d^7\) ions. Examples include \(\chem{Mn^{3+}}\), \(\chem{Co^{2+}}\), and \(\chem{Cr^{2+}}\).
Concept Check
Between \(\chem{[Mn(CN)_6]^{3–}}\) and \(\chem{[Mn(CN)_6]^{4–}}\) which is more likely to be high spin? Why?
\(\chem{[Mn(CN)_6]^{4-}}\) is more likely to be high spin because the charge on the \(\chem{Mn}\) ion is 2+ while the charge on the \(\chem{Mn}\) ion is 3+ in the other complex. With a larger charge, there is bigger splitting between energy levels, meaning strong field, or low spin.
The Crystal Field Diagrams for Octahedral and Tetrahedral Complexes
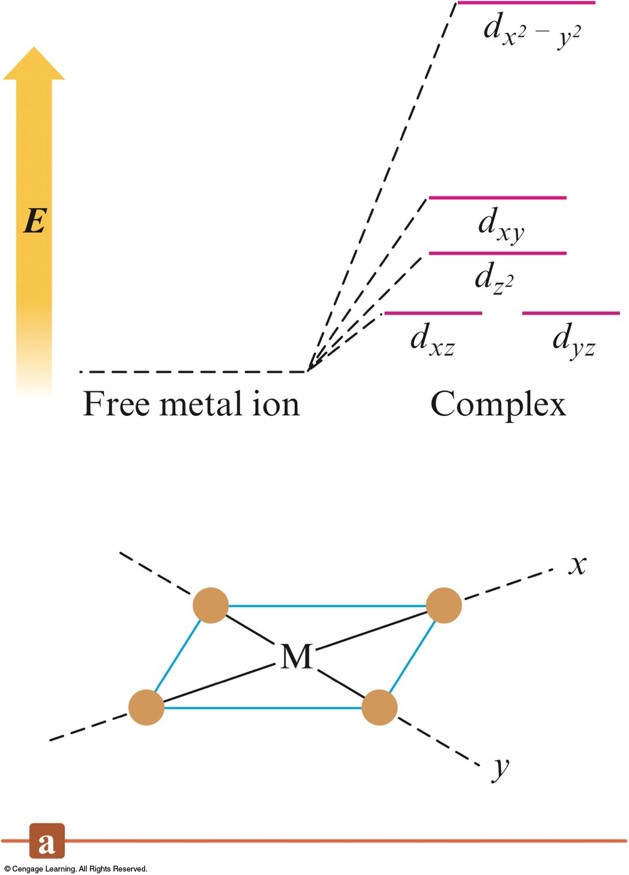
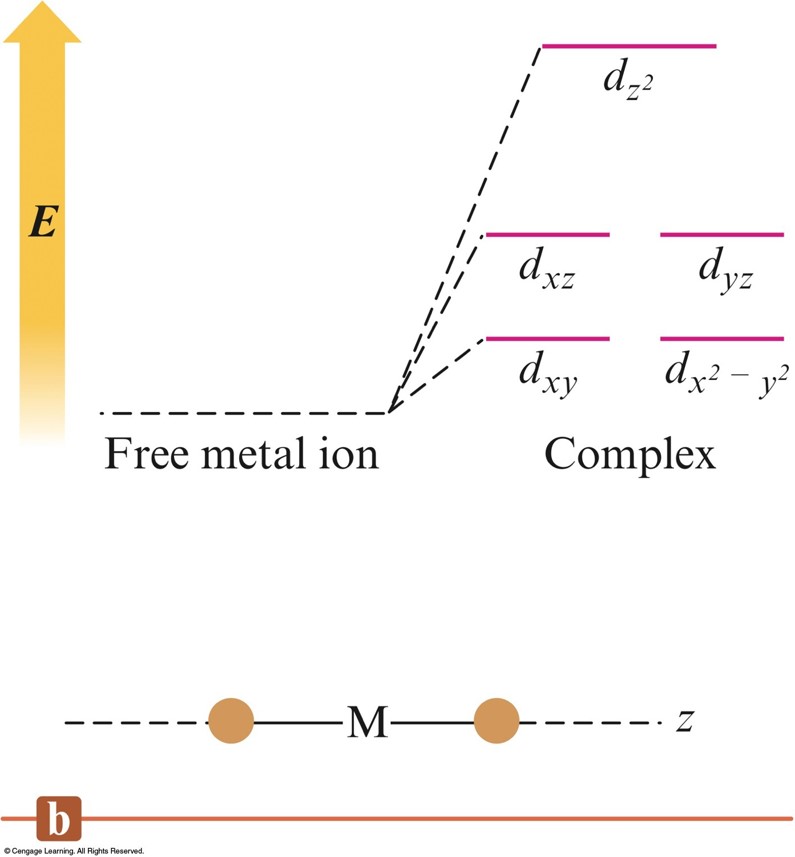
The \(d\) Energy Diagrams for Linear Complexes Where the Ligands Lie Along the \(z\) Axis
The Biological Importance of Coordination Complexes
- Metal ion complexes are used in humans for the transport and storage of oxygen, as electron-transfer agents, as catalysts, and as drugs.
First-Row Transition Metals and Their Biological Significance
| First-Row Transition Metals | Biological Function(s) |
|---|---|
| Scandium | None known. |
| Titanium | None known. |
| Vanadium | None known in humans. |
| Chromium | Assists insulin in the control of blood sugar; may also be involved in the control of cholesterol. |
| Manganese | Necessary for a number of enzymatic reactions. |
| Iron | Component of hemoglobin and myoglobin; involved in the electron-transport chain. |
| Cobalt | Component of vitamin B12, which is essential for the metabolism of carbohydrates, fats, and proteins. |
| Nickel | Component of the enzymes urease and hydrogenase. |
| Copper | Component of several enzymes; assists in iron storage; involved in the production of color pigments of hair, skin, and eyes. |
| Zinc | Component of insulin and many enzymes. |
Biological Importance of Iron
- Plays a central role in almost all living cells.
- Component of hemoglobin and myoglobin.
- Involved in the electron-transport chain.
The Heme Complex
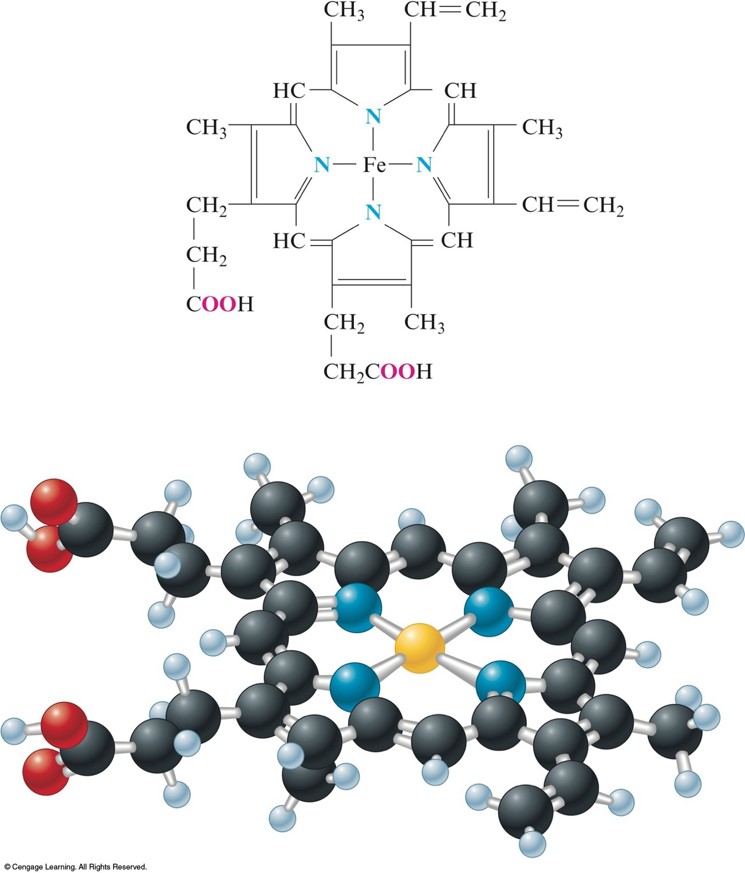
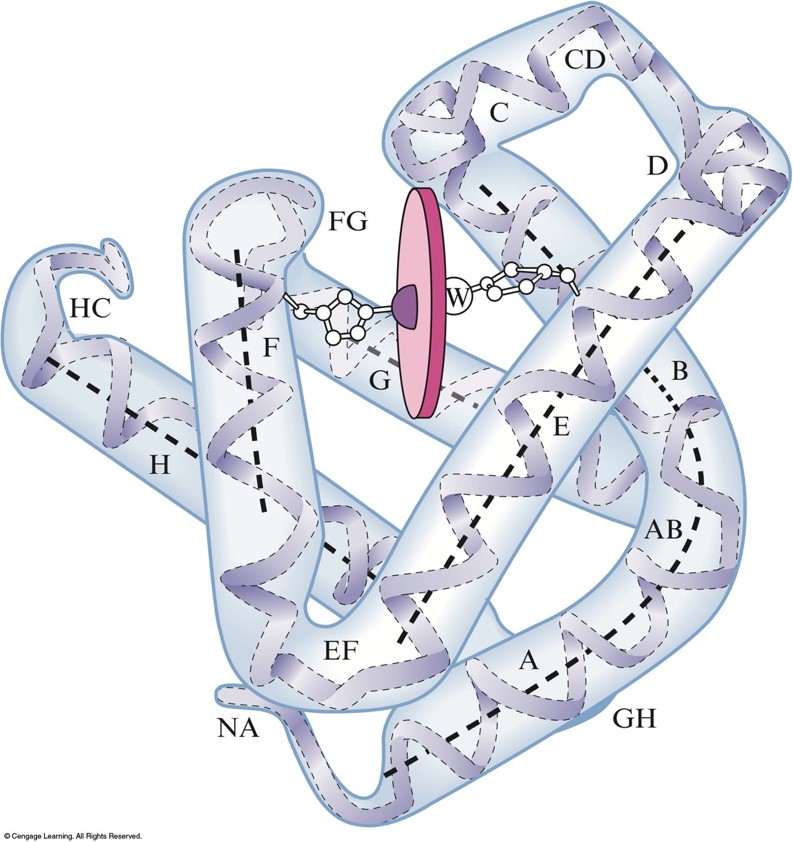
Myoglobin
- The \(\chem{Fe^{2+}}\) ion is coordinated to four nitrogen atoms in the porphyrin of the heme (the disk in the figure) and on nitrogen from the protein chain.
- This leaves a 6th coordination position (the \(\chem{W}\)) available for an oxygen molecule.
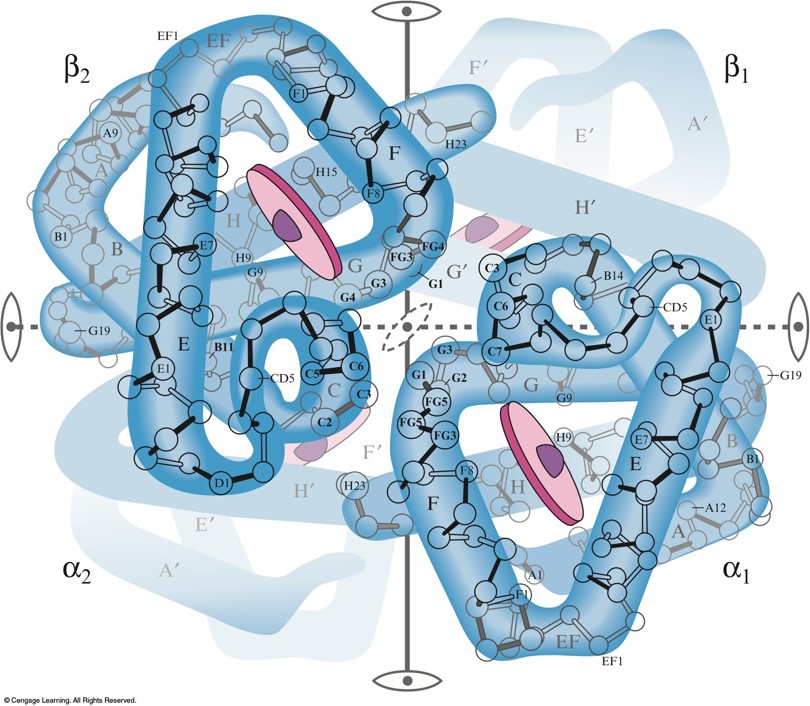
Hemoglobin
- Each hemoglobin has two \(\alpha\) chains and two \(\beta\) chains, each with a heme complex near the center.
- Each hemoglobin molecule can complex with four \(\chem{O_2}\) molecules.
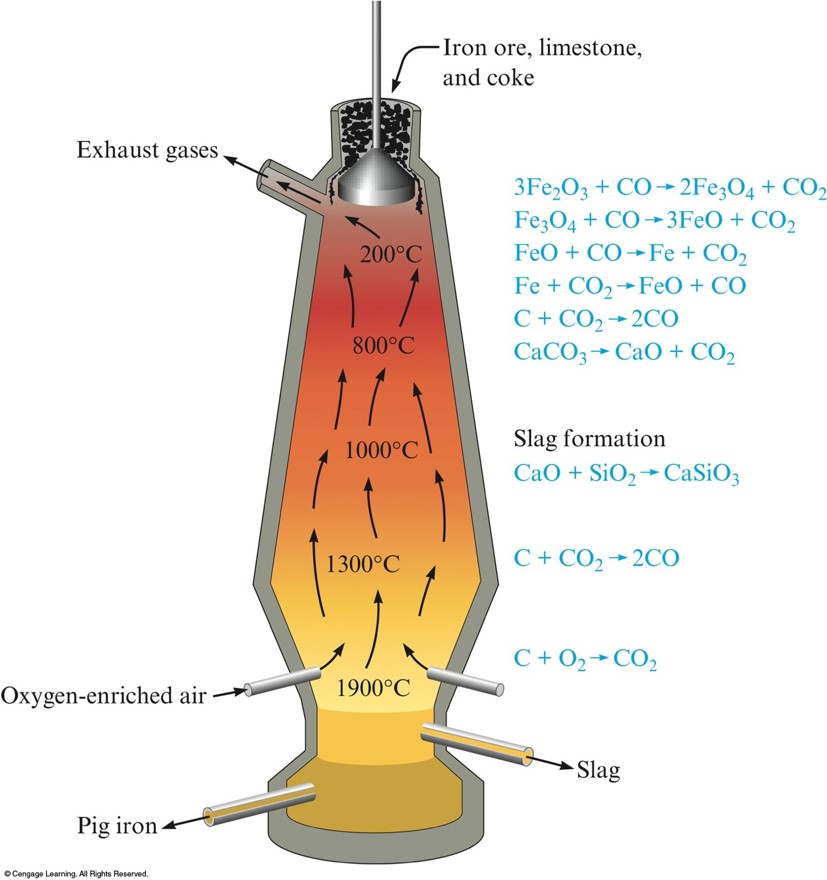
Metallurgy
- Process of separating a metal from its ore and preparing it for use.
- Steps:
- Mining
- Pretreatment of the ore
- Reduction to the free metal
- Purification of the metal (refining)
- Alloying
The Blast Furnace Used In The Production if Iron
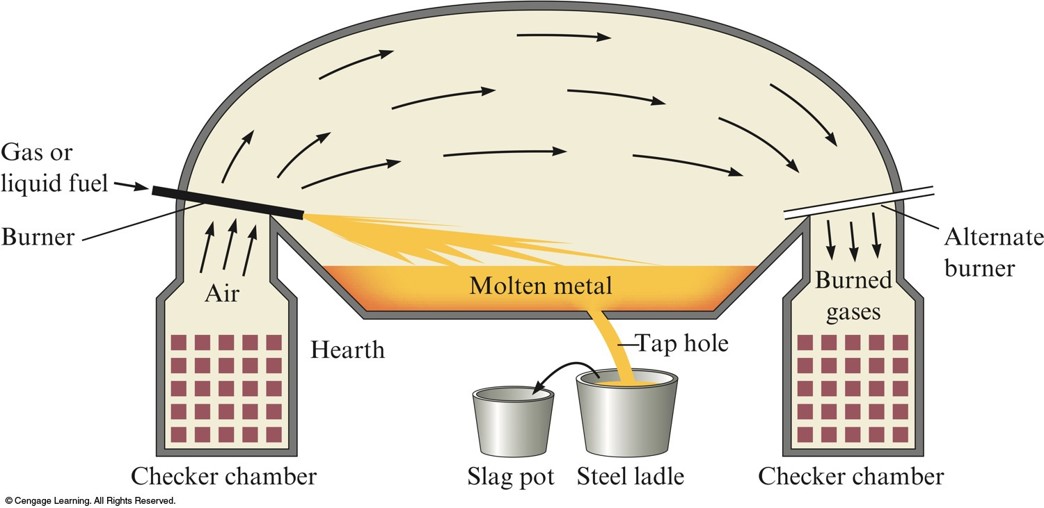
A Schematic of the Open Hearth Process for Steelmaking
$$ \begin{align} & \chem{CaCO_3 \xrightarrow{Heat} CaO+CO_2} \\ & \chem{4Al + 3O_2 \rightarrow 2Al_2O_3} \end{align} $$
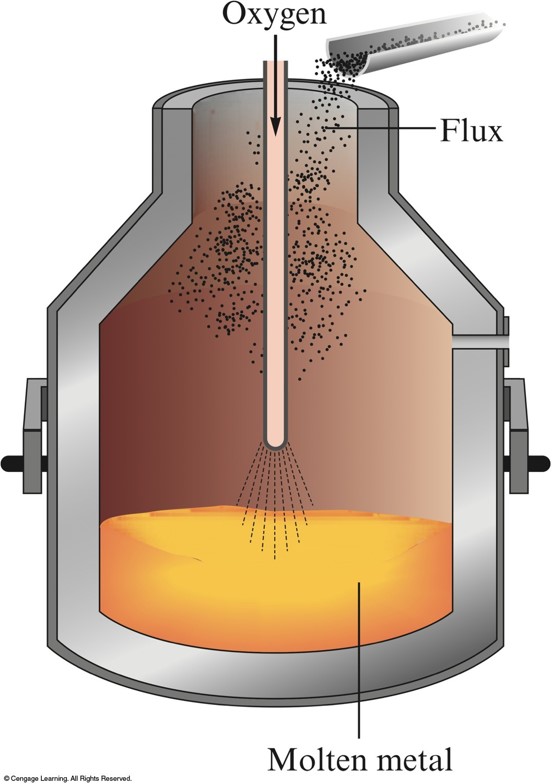
The Basic Oxygen Process for Steelmaking
- Much faster.
- Exothermic oxidation reactions proceed so rapidly that they produce enough heat to raise the temperature nearly to the boiling point of iron without an external heat source.
/