Chapter 17
Electrochemistry
Shaun Williams, PhD
Review of Terms
- Electrochemistry – the study of the interchange of chemical and electrical energy
- Oxidation–reduction (redox) reaction – involves a transfer of electrons from the reducing agent to the oxidizing agent
- Oxidation – loss of electrons
- Reduction – gain of electrons
- Reducing agent – electron donor
- Oxidizing agent – electron acceptor
Half-Reactions
- The overall reaction is split into two half–reactions, one involving oxidation and one involving reduction. $$ \begin{align} &\chem{8H^+ + MnO_4^- + 5Fe^{2+} \rightarrow Mn^{2+} + 5Fe^{3+} + 4H_2O} \\ &\text{Reduction: }\chem{8H^+ + MnO_4^- + 5e^- \rightarrow Mn^{2+} + 4H_2O} \\ &\text{Oxidation: }\chem{5Fe^{2+} \rightarrow 5Fe^{3+} + 5e^-} \end{align} $$
The Half–Reaction Method for Balancing Equations for Oxidation–Reduction Reactions Occurring in Acidic Solution
- Write separate equations for the oxidation and reduction half–reactions.
- For each half–reaction:
- Balance all the elements except \(\chem{H}\) and \(\chem{O}\).
- Balance \(\chem{O}\) using \(\chem{H_2O}\).
- Balance \(\chem{H}\) using \(\chem{H^+}\).
- Balance the charge using electrons.
- If necessary, multiply one or both balanced half–reactions by an integer to equalize the number of electrons transferred in the two half–reactions.
- Add the half–reactions, and cancel identical species.
- Check that the elements and charges are balanced.
Example
\(\chem{Cr_2O_7^{2-}(aq) + SO_3^{2-}(aq) \rightarrow Cr^{3+}(aq) + SO_4^{2-}(aq)}\)
- Seperate this into half-reactions $$ \begin{align} \chem{Cr_2O_7^{2-}(aq)} &\rightarrow \chem{Cr^{3+}(aq)} \\ \chem{SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq)} \end{align}$$
- Balance all the elements except \(\chem{H}\) and \(\chem{O}\) $$ \begin{align} \chem{Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq)} \\ \chem{SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq)} \end{align}$$
Example Continued
$$ \begin{align} \chem{Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq)} \\ \chem{SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq)} \end{align}$$
- Add electrons based on changes in oxidation states $$ \begin{align} \chem{6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{Cr^{3+}(aq)} \\ \chem{SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq) + 2e^-} \end{align}$$
- Balance the oxygen atoms using \(\chem{H_2O}\) $$ \begin{align} \chem{6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq) + 7H_2O} \\ \chem{H_2O + SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq) + 2e^-} \end{align}$$
Example Continued Further
$$ \begin{align} \chem{6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq) + 7H_2O} \\ \chem{H_2O + SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq) + 2e^-} \end{align}$$
- Balance the hydrogen atoms using \(\chem{H^+}\) $$ \begin{align} \chem{14H^+ + 6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq) + 7H_2O} \\ \chem{H_2O + SO_3^{2-}(aq)} &\rightarrow \chem{SO_4^{2-}(aq) + 2e^- + 2H^+} \end{align}$$
- Balance the electrons between the half-reactions $$ \begin{align} \chem{14H^+ + 6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq) + 7H_2O} \\ 3\left[\chem{H_2O + SO_3^{2-}(aq)}\right. &\rightarrow \left.\chem{SO_4^{2-}(aq) + 2e^- + 2H^+}\right] \end{align}$$
Example Concluded
$$ \begin{align} \chem{14H^+ + 6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq) + 7H_2O} \\ 3\left[\chem{H_2O + SO_3^{2-}(aq)}\right. &\rightarrow \left.\chem{SO_4^{2-}(aq) + 2e^- + 2H^+}\right] \end{align}$$
- Distributing $$ \begin{align} \chem{14H^+ + 6e^- + Cr_2O_7^{2-}(aq)} &\rightarrow \chem{2Cr^{3+}(aq) + 7H_2O} \\ 3\chem{H_2O + 3SO_3^{2-}(aq)} &\rightarrow \chem{3SO_4^{2-}(aq) + 6e^- + 6H^+} \end{align}$$
- Add the reactions together cancel out species on each side of the reaction to get the final balance equation $$ \chem{Cr_2O_7^{2-} + 3SO_3^{2-} + 8H^+ \rightarrow 2Cr^{3+} + 3SO_4^{2-} + 4H_2O} $$
Exercise
Balance the following oxidation-reduction reaction that occurs in acidic solution $$ \chem{Br^-(aq) + MnO_4^-(aq) \rightarrow Br_2(l) + Mn^{2+}(aq)} $$
$$ \begin{align} \chem{10Br^-(aq)} + \chem{16H^+(aq)} + \chem{2MnO_4^-(aq)} \rightarrow \\ \chem{5Br_2(l)} + \chem{2Mn^{2+}(aq)} + \chem{8H_2O(l)} \end{align} $$
The Half–Reaction Method for Balancing Equations for Oxidation–Reduction Reactions Occurring in Basic Solution
- Use the half–reaction method as specified for acidic solutions to obtain the final balanced equation as if \(\chem{H^+}\) ions were present.
- To both sides of the equation obtained above, add a number of \(\chem{OH^–}\) ions that is equal to the number of \(\chem{H^+}\) ions. (We want to eliminate \(\chem{H^+}\) by forming \(\chem{H_2O}\).)
- Form \(\chem{H_2O}\) on the side containing both \(\chem{H^+}\) and \(\chem{OH^–}\) ions, and eliminate the number of H2O molecules that appear on both sides of the equation.
- Check that elements and charges are balanced.
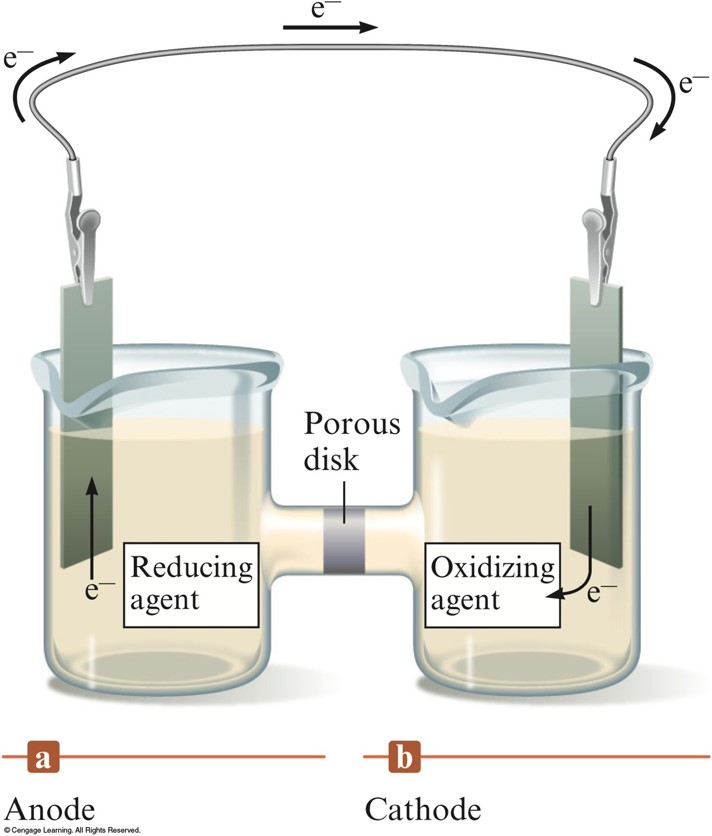
Galvantic Cell
- Device in which chemical energy is changed to electrical energy.
- Uses a spontaneous redox reaction to produce a current that can be used to do work.
- Oxidation occurs at the anode.
- Reduction occurs at the cathode.
- Salt bridge or porous disk – devices that allow ions to flow without extensive mixing of the solutions.
- Salt bridge – contains a strong electrolyte held in a Jello–like matrix.
- Porous disk – contains tiny passages that allow hindered flow of ions.
The Galvantic Cell
Cell Potential
- A galvanic cell consists of an oxidizing agent in one compartment that pulls electrons through a wire from a reducing agent in the other compartment.
- The “pull”, or driving force, on the electrons is called the cell potential (\(\mathcal{E}_{cell}\)), or the electromotive force (emf) of the cell.
- Unit of electrical potential is the volt (V).
- 1 joule of work per coulomb of charge transferred.
More on Galvanic Cells
- All half-reactions are given as reduction processes in standard tables.
- Table 18.1
- \(1\,\mathrm{M}\), \(1\,\mathrm{atm}\), \(25^\circ\mathrm{C}\)
- When a half-reaction is reversed, the sign of \(E^\circ\) is reversed.
- When a half-reaction is multiplied by an integer, \(E^\circ\) remains the same.
- A galvanic cell runs spontaneously in the direction that gives a positive value for \(E^\circ_{cell}\).
Example: \(\chem{Fe^{3+}(aq) + Cu(s) → Cu^{2+}(aq) + Fe^{2+}(aq)}\)
- Half-Reactions: $$ \begin{align} &\chem{Fe^{3+}+e^- \rightarrow Fe^{2+}} & E^\circ = 0.77\,\mathrm{V} \\ &\chem{Cu^{2+}+2e^- \rightarrow Cu} & E^\circ = 0.34\,\mathrm{V} \\ \end{align} $$
- To balance the cell reaction and calculate the cell potential, we must reverse reaction 2. $$ \chem{Cu \rightarrow Cu^{2+} + 2e^-} \;\;\;\; E^\circ = -0.34\,\mathrm{V} $$
- Each \(\chem{Cu}\) atom produces two electrons but each \(\chem{Fe^{3+}}\) ion accepts only one electron, therefore reaction 1 must be multiplied by 2. $$ \chem{2Fe^{3+} + 2e^- \rightarrow 2Fe^{2+}} \;\;\;\; E^\circ = 0.77\,\mathrm{V} $$
Overall Balanced Cell Reaction
- Balanced Cell Reaction: $$ \chem{Cu + 2Fe^{3+} \rightarrow Cu^{2+} + 2Fe^{2+}} $$
- Cell Potential: $$ \begin{align} E^\circ_{cell} &= E^\circ (\mathrm{cathode}) - E^\circ (\mathrm{anode}) \\ E^\circ_{cell} &= 0.77\,\mathrm{V} - 0.34\,\mathrm{V} = 0.43\,\mathrm{V} \end{align} $$
$$ \begin{align} &\chem{2Fe^{3+}+2e^- \rightarrow 2Fe^{2+}} & E^\circ = 0.77\,\mathrm{V} \\ &\chem{Cu \rightarrow Cu^{2+}+2e^-} & E^\circ = -0.34\,\mathrm{V} \\ \end{align} $$
Line Notation
- Used to describe electrochemical cells.
- Anode components are listed on the left.
- Cathode components are listed on the right.
- Separated by double vertical lines which indicated salt bridge or porous disk.
- The concentration of aqueous solutions should be specified in the notation when known.
- Example: \( \chem{Mg(s) | Mg^{2+}(aq) || Al^{3+}(aq) | Al(s)} \)
- \( \chem{Mg \rightarrow Mg^{2+} + 2e^-}\;\;\;\mathrm{(anode)} \)
- \( \chem{Al^{3+} + 3e^- \rightarrow Al}\;\;\;\;\;\mathrm{(cathode)} \)
Description of a Galvanic Cell
- The cell potential (always positive for a galvanic cell where \(E^\circ_{cell} = E^\circ (\mathrm{cathode}) – E^\circ (\mathrm{anode})\) and the balanced cell reaction.
- The direction of electron flow, obtained by inspecting the half–reactions and using the direction that gives a positive \(E^\circ_{cell}\).
- Designation of the anode and cathode.
- The nature of each electrode and the ions present in each compartment. A chemically inert conductor is required if none of the substances participating in the half–reaction is a conducting solid.
Work
- Work is never the maximum possible if any current is flowing.
- In any real, spontaneous process some energy is always wasted – the actual work realized is always less than the calculated maximum.
Maximum Cell Potential
- Directly related to the free energy difference between the reactants and the products in the cell.
- \( \Delta G^\circ = –nFE^\circ \)
- \( F = 96485\,\bfrac{\mathrm{C}}{\mathrm{mol\cdot e^-}} \)
A Concentration Cell
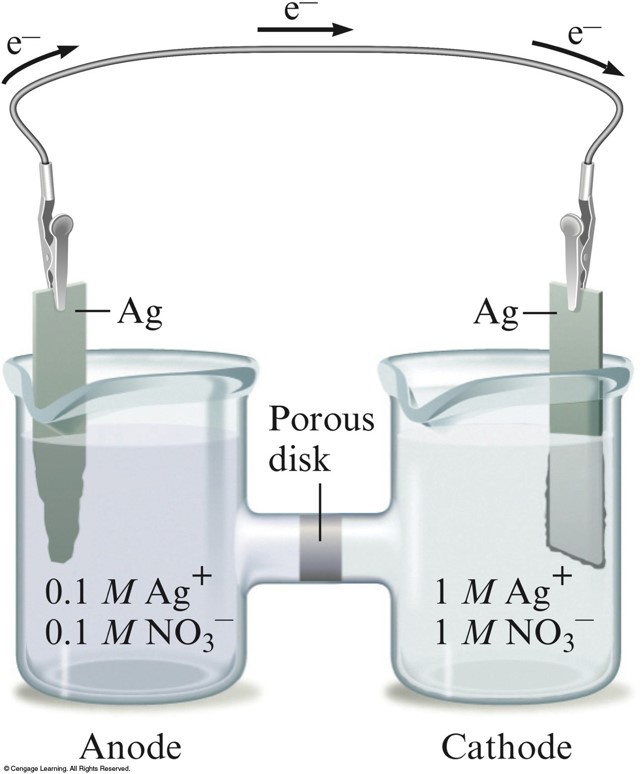
Nernst Equation
- The relationship between cell potential and concentrations of cell components
- At \(25^\circ\mathrm{C}\): $$ \begin{align} \mathcal{E} &= \mathcal{E}^\circ - \frac{0.0591}{n} \log (Q) \\ &\mathrm{or} \\ \mathcal{E}^\circ &= \frac{0.0591}{n}\log (K) \;\;\; \text{(at equilibrium)} \end{align} $$
Exercise
A concentration cell is constructed using two nickel electrodes with \(\chem{Ni^{2+}}\) concentrations of \(1.0\,\mathrm{M}\) and \(1.00 \times 10^{-4}\,\mathrm{M}\) in the two half-cells.
Calculate the potential of this cell at \(25^\circ\mathrm{C}\).
\(0.118\,\mathrm{V}\)
Concept Check
You make a galvanic cell at \(25^\circ\mathrm{C}\) containing:
- A nickel electrode in \( 1.0\,\mathrm{M}\,\chem{Ni^{2+}(aq)} \)
- A silver electrode in \( 1.0\,\mathrm{M}\,\chem{Ag^+(aq)} \)
Sketch this cell, labeling the anode and cathode, showing the direction of the electron flow, and calculate the cell potential.
\( 1.03\,\mathrm{V} \)
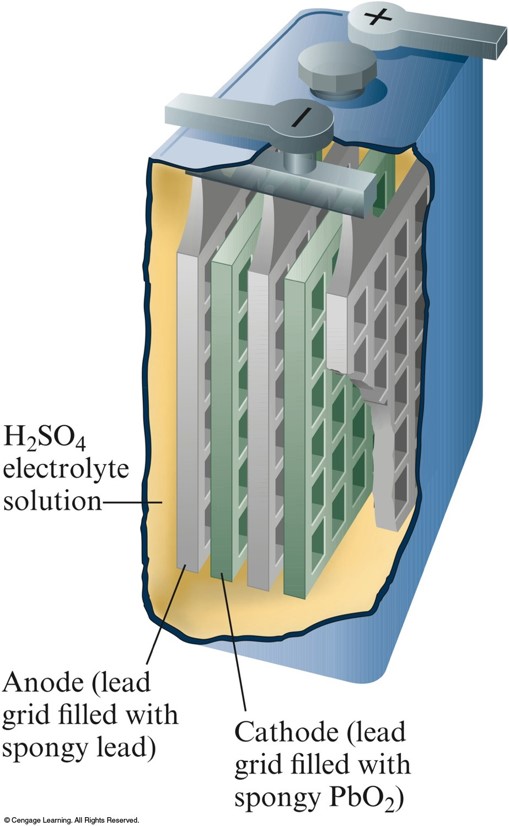
One of the Six Cells in a 12–V Lead Storage Battery
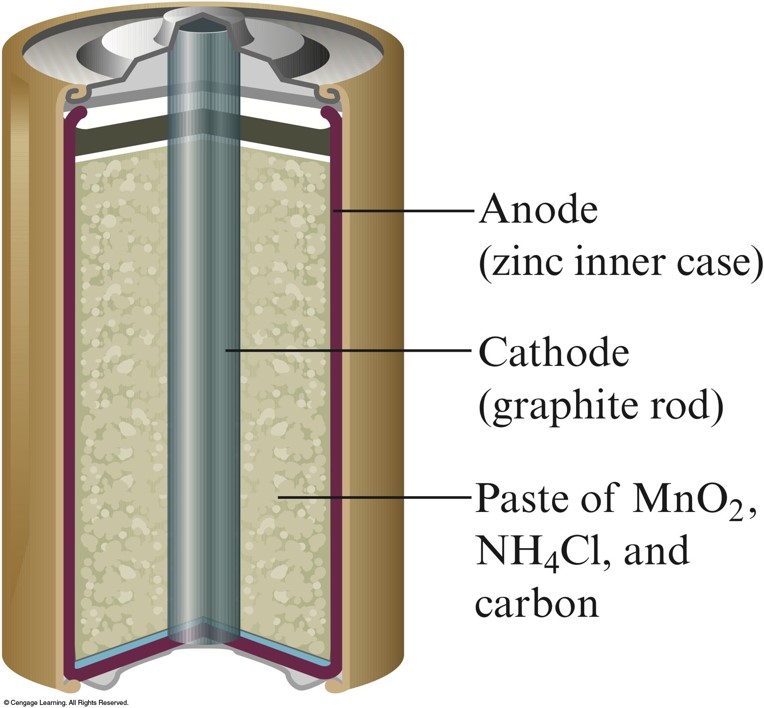
A Common Dry Cell Battery
A Mercury Battery
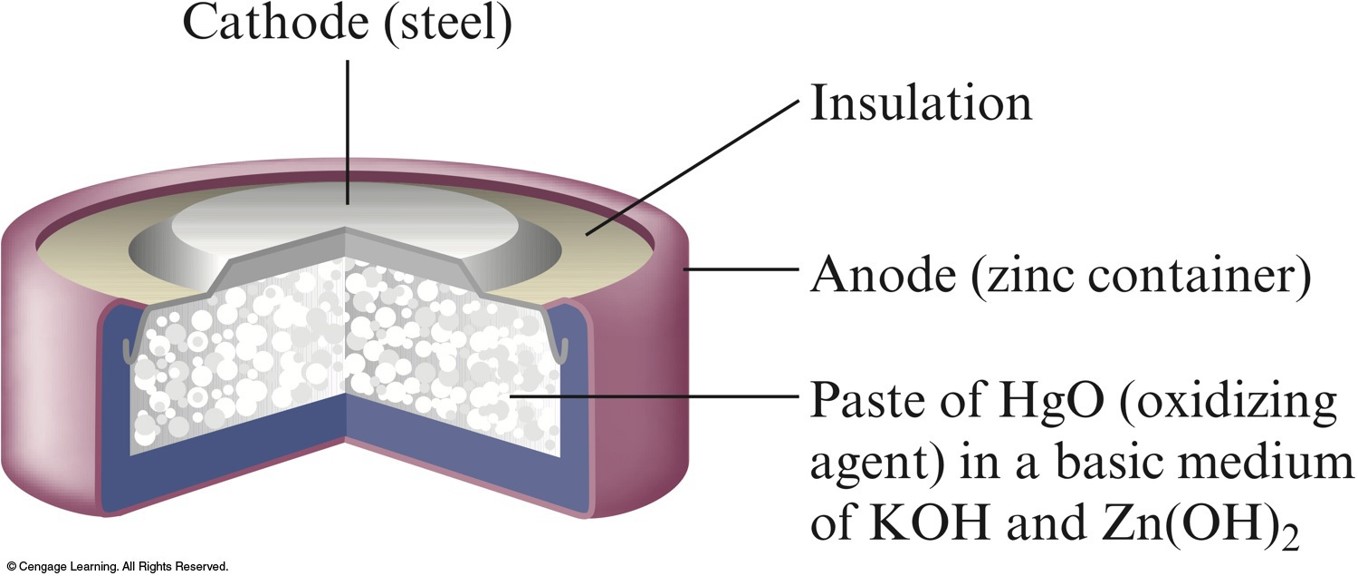
Schematic of the Hydrogen-Oxygen Fuel Cell
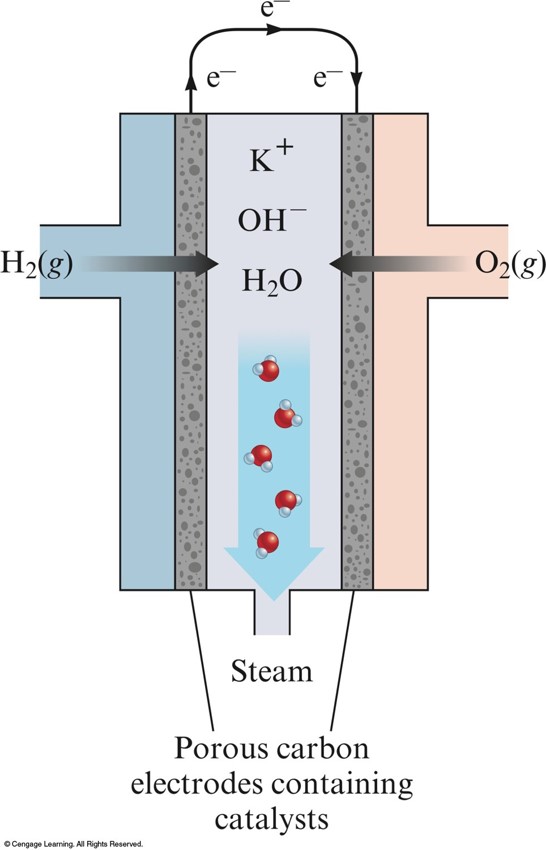
Corrosion
- Process of returning metals to their natural state – the ores from which they were originally obtained.
- Involves oxidation of the metal.
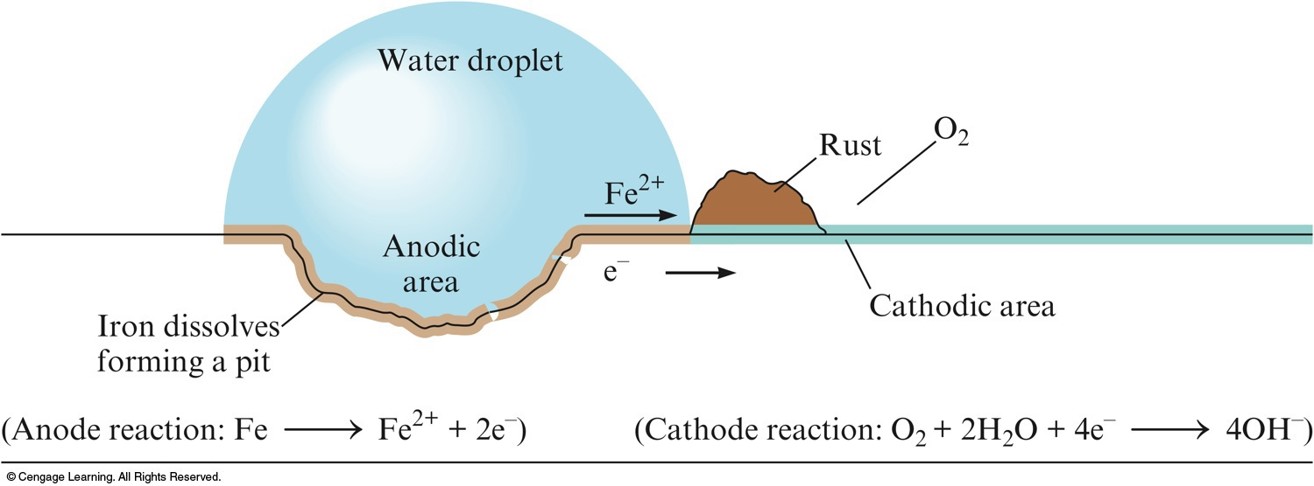
Corrosion Prevention
- Application of a coating (like paint or metal plating)
- Galvanizing
- Alloying
- Cathodic Protection
- Protects steel in buried fuel tanks and pipelines.
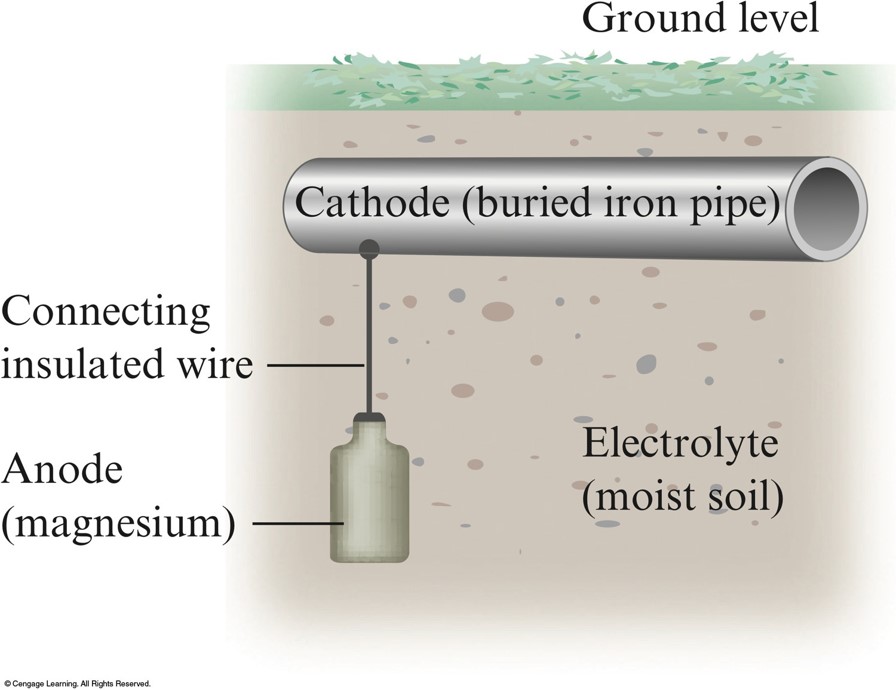
Cathodic Protection
Electrolysis
- Forcing a current through a cell to produce a chemical change for which the cell potential is negative.
- Steps of stoichiometry of electrolysis
- current and time \(\rightarrow\) quantity of charge $$ \text{Coulombs of charge} = \text{current (C/s)} \times \text{time (s)} $$
- quantity of charge \(\rightarrow\) moles of electrons $$ \text{mole }e^- = \text{Coulombs of charge} \times \frac{1\,\mathrm{mol}\,e^-}{96485\,\mathrm{C}} $$
- moles of electrons \(\rightarrow\) moles of analyte
- moles of analyte \(\rightarrow\) grams of analyte
Concept Check
An unknown metal (\(\chem{M}\)) is electrolyzed. It took 52.8 sec for a current of 2.00 amp to plate 0.0719 g of the metal from a solution containing \(\chem{M\left(NO_3\right)_3}\).
What is the metal?
gold (Au)
Commercial Electrolytic Processes
- Production of aluminum
- Purification of metals
- Metal plating
- Electrolysis of sodium chloride
- Production of chlorine and sodium hydroxide
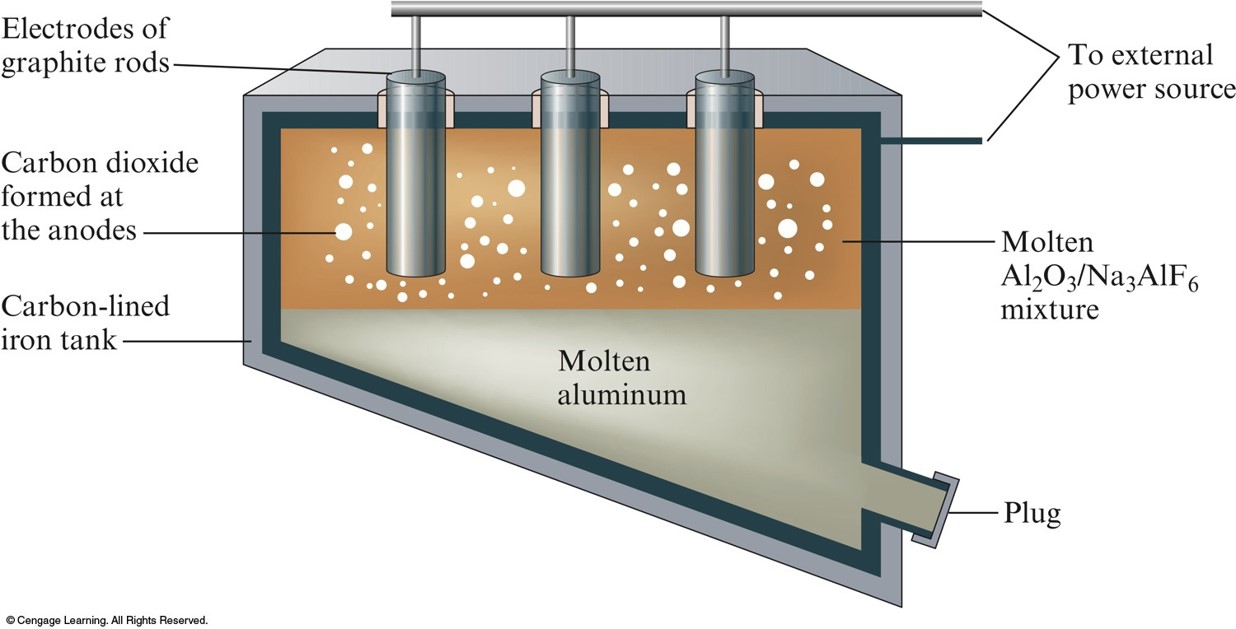
Producing Aluminum by the Hall-Heroult Process
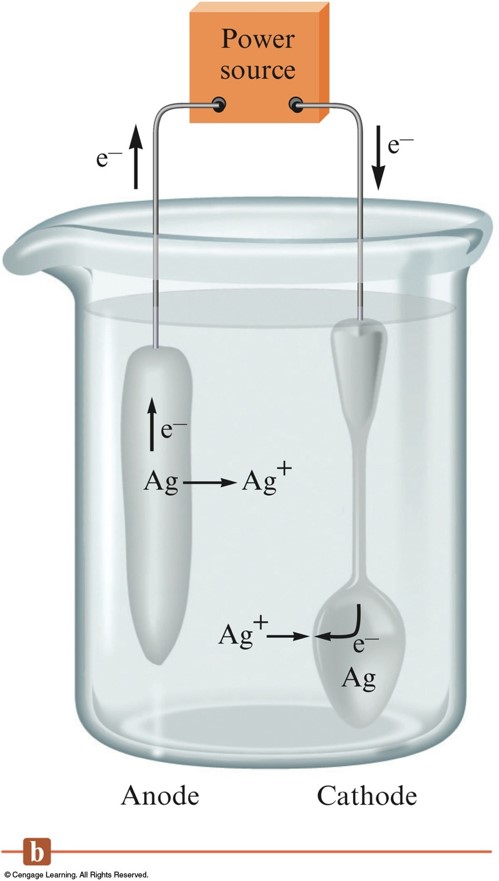
Electroplating a Spoon
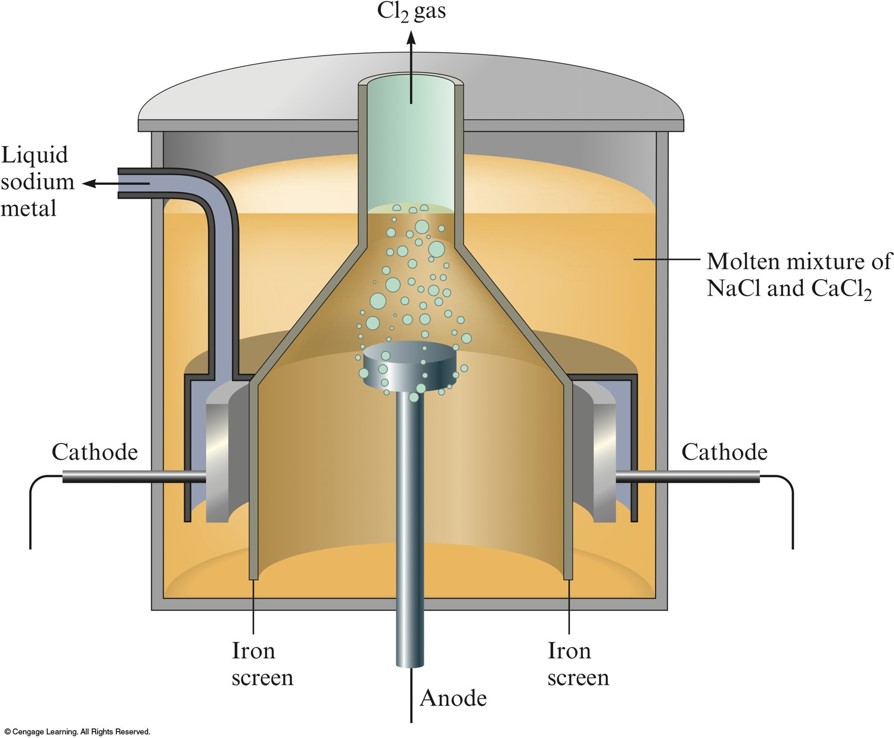
The Downs Cell for the Electrolysis of Molten Sodium Chloride
/