Chapter 16
Thermodynamics
Shaun Williams, PhD
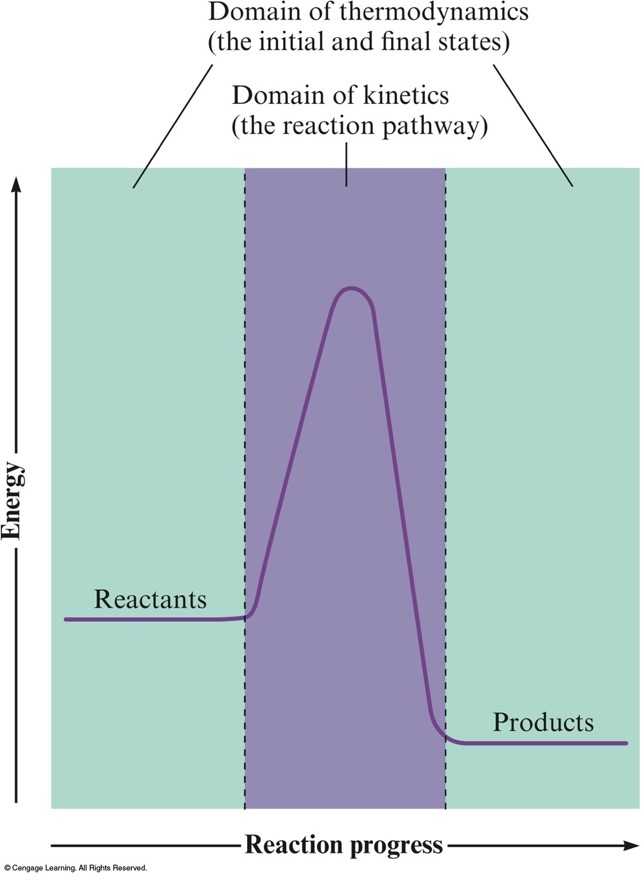
Thermodynamics vs. Kinetics
- Domain of Kinetics
- Rate of a reaction depends on the pathway from reactants to products.
- Thermodynamics tells us whether a reaction is spontaneous based only on the properties of reactants and products.
Spontaneous Processes and Entropy
- Thermodynamics lets us predict the direction in which a process will occur but gives no information about the speed of the process.
- A spontaneous process is one that occurs without outside intervention.
Concept Check
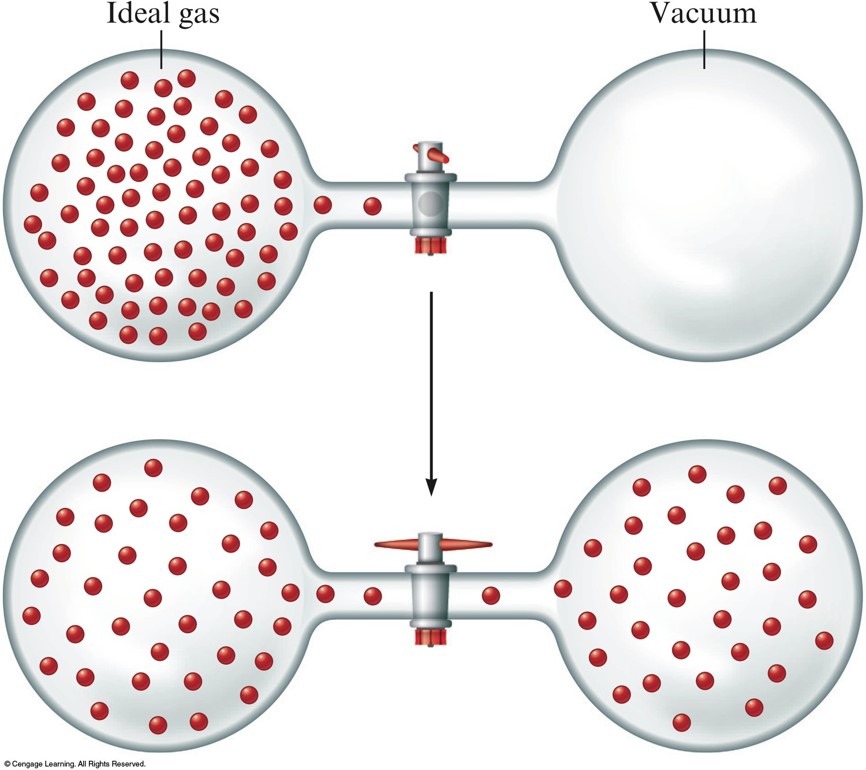
Consider \(2.4\,\mathrm{moles}\) of a gas contained in a \(4.0\,\mathrm{L}\) bulb at a constant temperature of \(32^\circ \mathrm{C}\). This bulb is connected by a valve to an evacuated \(20.0\,\mathrm{L}\) bulb. Assume the temperature is constant.
What should happen to the gas when you open the value?
The gas should spread evenly throughout the two bulbs.
The Expansion of an Ideal Gas Into an Evacuated Bulb
Entropy
- The driving force for a spontaneous process is an increase in the entropy of the universe.
- A measure of molecular randomness or disorder.
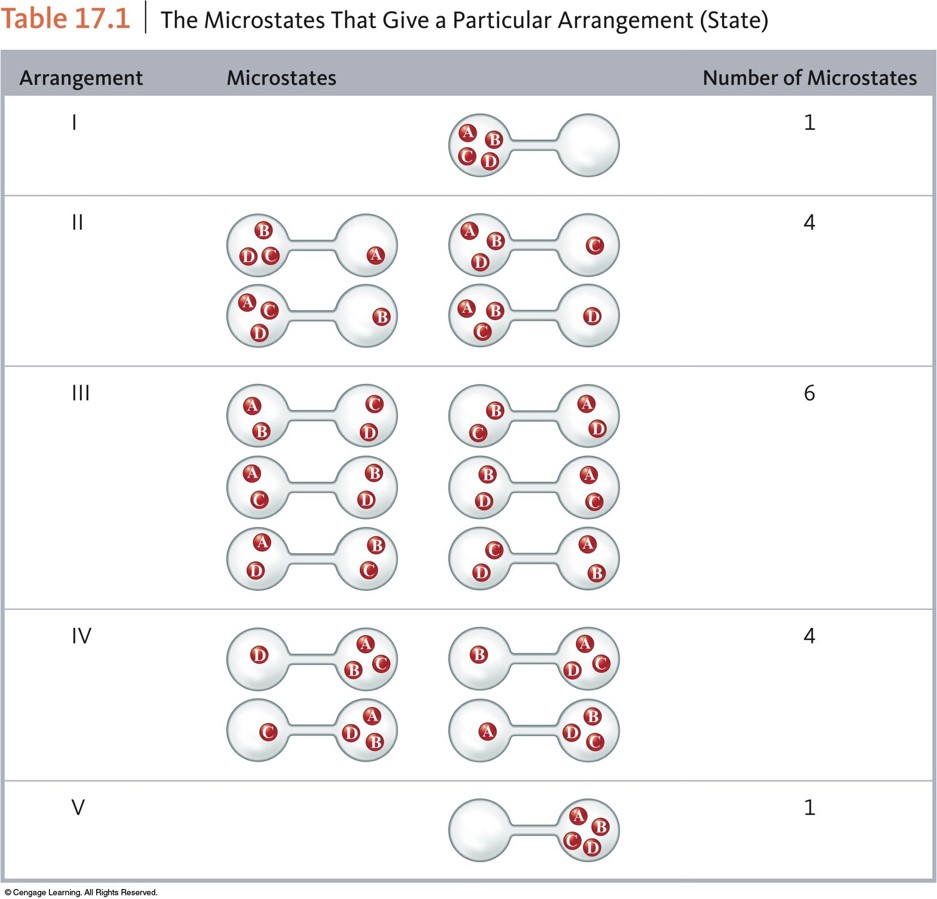
- Thermodynamic function that describes the number of arrangements that are available to a system existing in a given state.
- Nature spontaneously proceeds toward the states that have the highest probabilities of existing.
The Microstates That Give a Particular Arrangement (State)
Positional Entropy
- A gas expands into a vacuum to give a uniform distribution because the expanded state has the highest positional probability of states available to the system.
- Therefore: \(S_{solid} < S_{liquid} \ll S_{gas}\)

Concept Check
Predict the sign of \(\Delta S\) for each of the following:
- The evaporation of alcohol
- The freezing of water
- Compressing an ideal gas at constant temperature
- Heating an ideal gas at constant pressure
- Dissolving NaCl in water
positive
negative
negative
positive
positive
Second Law of Thermodynamics
- In any spontaneous process there is always an increase in the entropy of the universe.
- The entropy of the universe is increasing.
- The total energy of the universe is constant, but the entropy is increasing. $$ S_{universe}=\Delta S_{system}+\Delta S_{surroundings} $$
\(\Delta S_{surr}\)
- \(\Delta S_{surr} > 0\): entropy of the universe increases
- \(\Delta S_{surr} < 0\): process is spontaneous in opposite direction
- \(\Delta S_{surr} = 0\): process has no tendency to occur
- The sign of \(\Delta S_{surr}\) depends on the direction of the heat flow.
- The magnitude of \(\Delta S_{surr}\) depends on the temperature.
- Exothermic process: \( \Delta S_{surr} = +\frac{\text{quantity of heat (J)}}{\text{temperature (K)}} \)
- Endothermic process: \( \Delta S_{surr} = -\frac{\text{quantity of heat (J)}}{\text{temperature (K)}} \)
- Heat flow (constant \(P\)) = change in enthalpy = \(\Delta H\) $$\Delta S_{surr}=-\frac{\Delta H}{T}$$
Interplay of \(\Delta S_{sys}\) and \(\Delta S_{surr}\) in Determining the Sign of \(\Delta S_{univ}\)
| Signs of Entropy Changes | |||
|---|---|---|---|
| \(\Delta S_{sys}\) | \(\Delta S_{surr}\) | \(\Delta S_{univ}\) | Process Spontaneous? |
| + | + | + | Yes |
| - | - | - | No (reaction will occur in opposite direction) |
| + | - | ? | Yes, if \(\Delta S_{sys}\) has a larger magnitude than \(\Delta S_{surr}\) |
| - | + | ? | Yes, if \(\Delta S_{surr}\) has a larger magnitude than \(\Delta S_{sys}\) |
Free Energy (\(G\))
$$ \Delta S_{univ} = -\frac{\Delta G}{T}\,\left( \text{at constant }T\text{ and }P \right) $$
- A process (at constant \(T\) and \(P\) is spontaneous in the direction in which the free energy decreases.
- Negative \(\Delta G\) means positive \(\Delta S_{univ}\).
- \( \Delta G = \Delta H - T\Delta S \) (at constant \(T\) and \(P\))
Effect of \(\Delta H\) and \(\Delta S\) on Spontaneity
| Case | Result |
|---|---|
| \(\Delta S > 0\), \(\Delta H <0\) | Spontaneous at all temperatures |
| \(\Delta S > 0\), \(\Delta H > 0\) | Spontaneous at high temperatures (where exothermicity is relatively unimportant) |
| \(\Delta S < 0\), \(\Delta H < 0\) | Spontaneous at low temperatures (where exothermicity is dominant) |
| \(\Delta S < 0\), \(\Delta H > 0\) | Process not spontaneous at any temperatures (reverse process is spontaneous at all temperatures) |
Third Law of Thermodynamics
- The entropy of a perfect crystal at \(0\,\mathrm{K}\) is zero.
- The entropy of a substance increases with temperature.
Standard State Values
- Standard Entropy Values (\(S^\circ\))
- Represent the increase in entropy that occurs when a substance is heated from \(0\,\mathrm{K}\) to \(298\,\mathrm{K}\) at 1 atm pressure. $$ \Delta S^\circ_{rxn} = \sum n_pS^\circ_{products} - \sum n_r S^\circ_{reactants} $$
- Standard Free Energy Values (\(\Delta G^\circ\))
- The change in free energy that will occur if the reactants in their standard states are converted to the products in their standard states. $$ \Delta G^\circ = \Delta H^\circ - T\Delta S^\circ $$ $$ \Delta G^\circ_{rxn} = \sum n_p G^\circ_{products} - \sum n_r G^\circ_{reactants} $$
Concept Check
A stable diatomic molecule spontaneously forms from its atoms. Predict the sign of
- \(\Delta H^\circ\)
- \(\Delta S^\circ\)
- \(\Delta G^\circ\)
negative
negative
negative
Free Energy
- Free energy and pressure $$ \begin{align} G &= G^\circ + RT\ln (P) \\ &\mathrm{or} \\ \Delta G &= \Delta G^\circ + RT\ln (Q) \end{align} $$
- Free energy and equilibrium $$ \Delta G=0=\Delta G^\circ + RT\ln (K) $$ $$ \Delta G^\circ = -RT \ln (K) $$
The Meaning of \(\Delta G\) for a Chemical Reaction
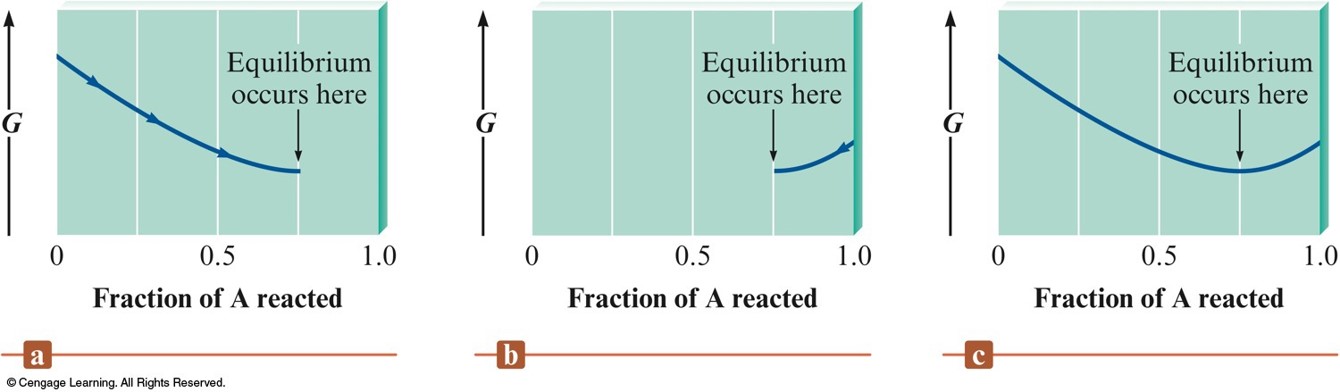
- A system can achieve the lowest possible free energy by going to equilibrium, not by going to completion.
Change in Free Energy to Reach Equilibrium
Qualitative Relationship Between the Change in Standard Free Energy and the Equilibrium Constant for a Given Reaction
| \(\Delta G^\circ\) | \(K\) |
|---|---|
| \(\Delta G^\circ = 0\) | \(K=1\) |
| \(\Delta G^\circ < 0\) | \(K>1\) |
| \(\Delta G^\circ > 0\) | \(K<1\) |
Free Energy and Work
- Maximum possible useful work obtainable from a process at constant temperature and pressure is equal to the change in free energy. $$ w_{max} = \Delta G $$
- Achieving the maximum work available from a spontaneous process can occur only via a hypothetical pathway. Any real pathway wastes energy.
- All real processes are irreversible.
- First law: You can’t win, you can only break even.
- Second law: You can’t break even.
- As we use energy, we degrade its usefulness.
/